Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization Energy (Ei)
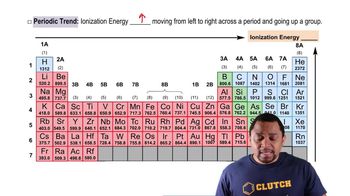
Ionization energy is the energy required to remove an electron from an atom or ion in its gaseous state. It is a critical concept in understanding how easily an atom can lose electrons, which is influenced by factors such as atomic size, nuclear charge, and electron shielding. Generally, ionization energy increases across a period and decreases down a group in the periodic table.
Recommended video:
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. It provides insight into the atom's chemical properties and reactivity. The configurations given in the question indicate the number of electrons in each energy level and sublevel, which directly affects the atom's ionization energies and how tightly electrons are held by the nucleus.
Recommended video:
Electron Configuration Example
Trends in Ionization Energy
Trends in ionization energy refer to the predictable changes in ionization energy values across periods and groups in the periodic table. As you move from left to right across a period, ionization energy generally increases due to increased nuclear charge and decreased atomic radius. Conversely, as you move down a group, ionization energy decreases because of increased electron shielding and greater distance from the nucleus, making it easier to remove electrons.
Recommended video:
 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 62
Problem 62 Verified step by step guidance
Verified step by step guidance