Magnesium, the element, is produced commercially by electrolysis from a molten salt (the 'electrolyte') using a cell similar to the one shown here. (b) Chlorine gas is evolved as voltage is applied in the cell. Knowing this, identify the electrolyte.
Ch.20 - Electrochemistry
Chapter 20, Problem 13b
(b) On which side of an oxidation half-reaction do the electrons appear?
 Verified step by step guidance
Verified step by step guidance1
Understand that an oxidation half-reaction involves the loss of electrons from a species.
Recall that in a chemical equation, reactants are written on the left side and products on the right side.
In an oxidation half-reaction, the species losing electrons is the reactant.
Electrons are considered products in an oxidation half-reaction because they are released by the reactant.
Therefore, in an oxidation half-reaction, electrons appear on the right side of the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
28sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation and Reduction
Oxidation and reduction are chemical processes that involve the transfer of electrons between substances. Oxidation refers to the loss of electrons, while reduction involves the gain of electrons. These processes are coupled, meaning that when one substance is oxidized, another is reduced. Understanding these concepts is essential for identifying the roles of reactants in redox reactions.
Recommended video:
Guided course

Oxidation and Reduction Reactions
Half-Reactions
Half-reactions are a way to represent oxidation and reduction processes separately. In a half-reaction, the species undergoing oxidation or reduction is shown along with the electrons involved in the process. This method simplifies the analysis of redox reactions by allowing chemists to focus on the electron transfer without the complexity of the entire reaction. Each half-reaction can be balanced independently.
Recommended video:
Guided course

First-Order Half-Life
Electron Placement in Half-Reactions
In an oxidation half-reaction, electrons are shown on the right side of the equation, indicating that they are being produced or released as a result of the oxidation process. This placement reflects the loss of electrons by the oxidized species. Conversely, in a reduction half-reaction, electrons appear on the left side, signifying their gain by the reduced species. Understanding this placement is crucial for correctly interpreting redox reactions.
Recommended video:
Guided course
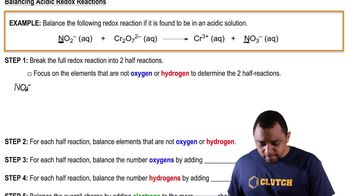
Redox Half Reactions Example
Related Practice
Textbook Question
871
views
1
rank
Textbook Question
Magnesium, the element, is produced commercially by electrolysis from a molten salt (the 'electrolyte') using a cell similar to the one shown here. (c) Recall that in an electrolytic cell the anode is given the + sign and the cathode is given the – sign, which is the opposite of what we see in batteries. What half-reaction occurs at the anode in this electrolytic cell?
848
views
Open Question
(a) What is meant by the term oxidation? (b) What is meant by the term oxidant? (c) What is meant by the term oxidizing agent?
Open Question
(a) What is meant by the term reduction? (b) On which side of a reduction half-reaction do the electrons appear? (c) What is meant by the term reductant? (d) What is meant by the term reducing agent?
Textbook Question
Indicate whether each of the following statements is true or false: (a) If something is oxidized, it is formally losing electrons.
576
views
Textbook Question
Indicate whether each of the following statements is true or false: (b) For the reaction Fe3+(aq) + Co2+(aq) → Fe2+(aq) + Co3+(aq), Fe3+(aq) is the reducing agent and Co2+(aq) is the oxidizing agent.
