The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (b) A sample consisting of various gases contains 3.5 × 10-6 mole fraction of radon. This gas at a total pressure of 32 atm is shaken with water at 30 °C. Calculate the molar concentration of radon in the water.
The first stage of treatment at a reverse osmosis plant is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Filtration
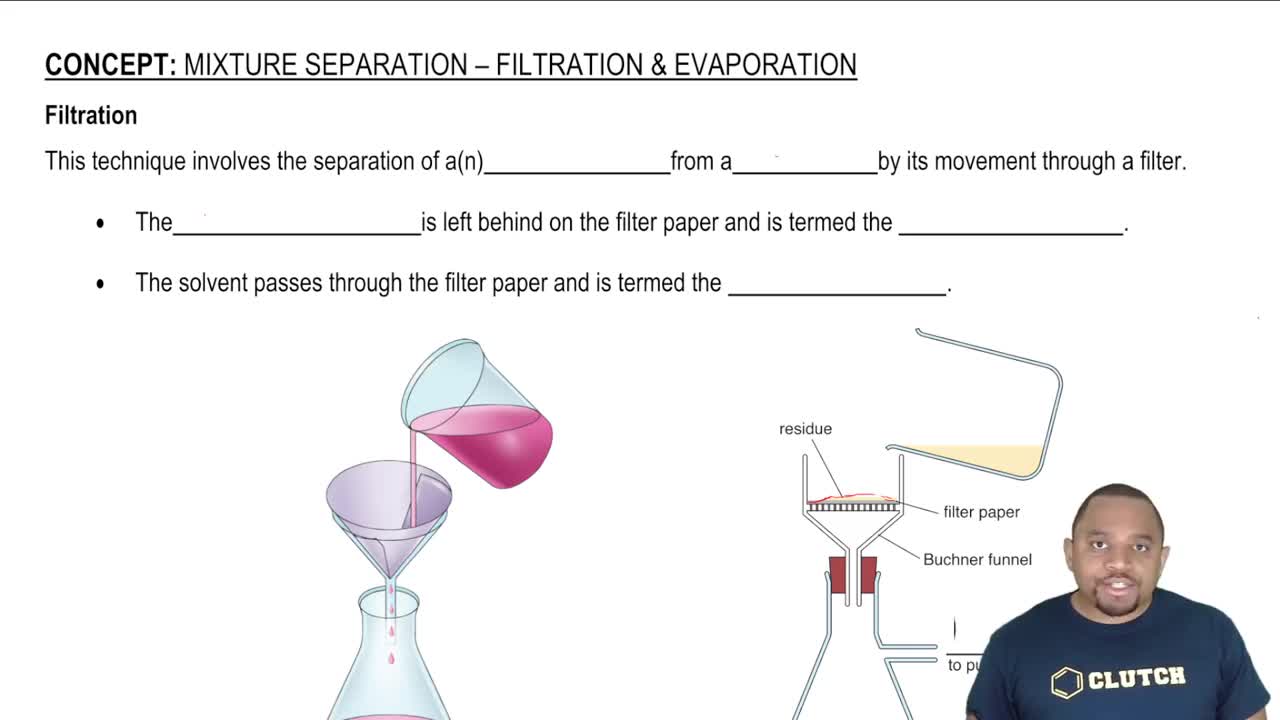
Dissolved Solids

Reverse Osmosis

The maximum allowable concentration of lead in drinking water is 9.0 ppb. (a) Calculate the molarity of lead in a 9.0-ppb solution.
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (b) How many grams of lead are in a swimming pool containing 9.0 ppb lead in 60 m3 of water?
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (a) molality,
Acetonitrile (CH3CN) is a polar organic solvent that dissolves a wide range of solutes, including many salts. The density of a 1.80 M LiBr solution in acetonitrile is 0.826 g/cm3. Calculate the concentration of the solution in (b) mole fraction of LiBr,
Two beakers are placed in a sealed box at 25 °C. One beaker contains 30.0 mL of a 0.050 M aqueous solution of a nonvolatile nonelectrolyte. The other beaker contains 30.0 mL of a 0.035 M aqueous solution of NaCl. The water vapor from the two solutions reaches equilibrium. (a) In which beaker does the solution level rise, and in which one does it fall?
