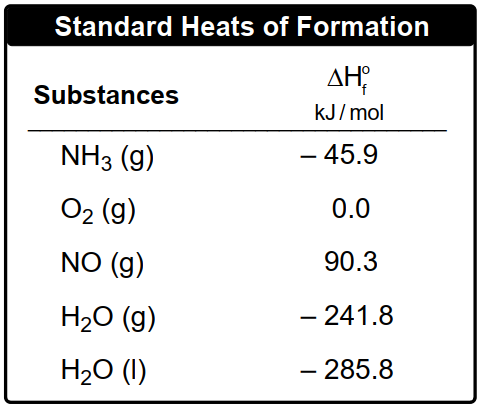
Consider the following equation:
2 ClF3(g) + 2 NH3(g) → 1 N2(g) + 6 HF (g) + 6 Cl2(g) ΔHrxn = –1196 kJ
Determine the standard enthalpy of formation for chlorine trifluoride, ClF3.
-906 kJ
-1273.2 kJ
1089.6 kJ
-183.6 kJ

 2:34m
2:34mMaster Enthalpy of Formation with a bite sized video explanation from Jules Bruno
Start learning
