Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 119.3 kPa and 24 °C. (c) At what temperature will the volume be 15.00 L if the pressure is 116.8 kPa
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ideal Gas Law

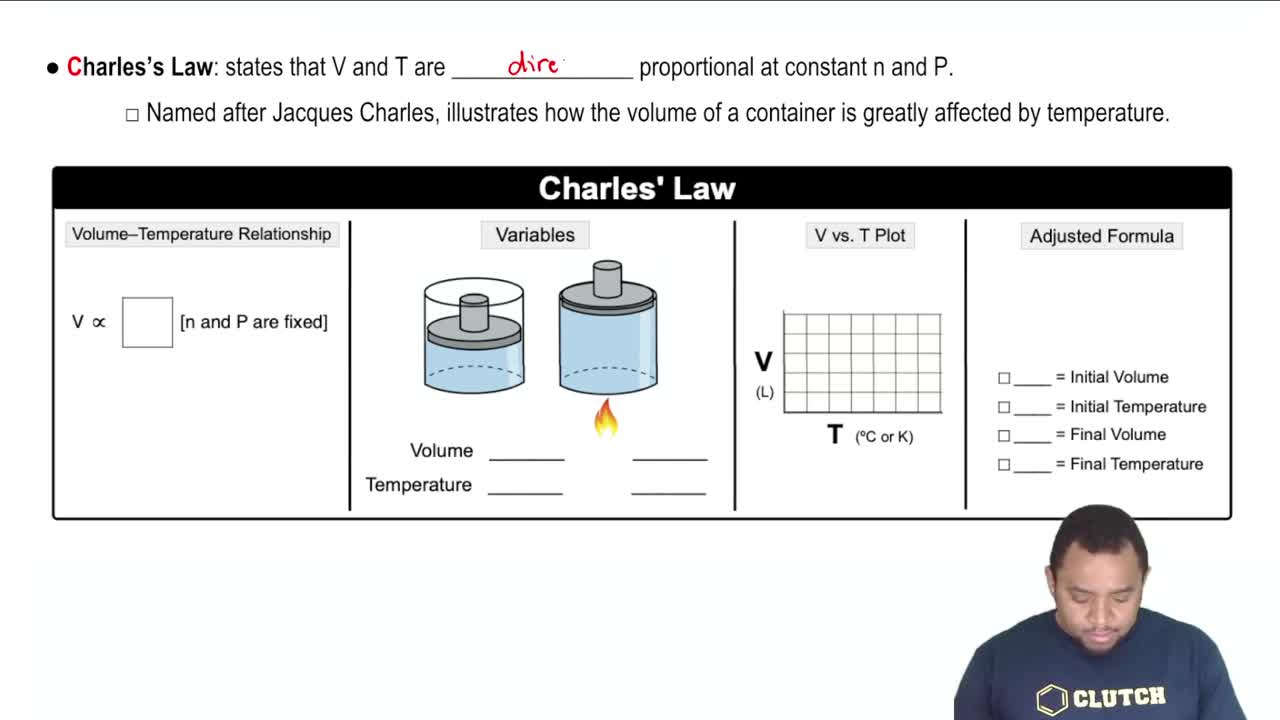
Charles's Law
Pressure-Volume Relationship

Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 119.3 kPa and 24 °C. (b) What volume will the Cl2 occupy at STP?
Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 119.3 kPa and 24 °C. (d) At what pressure will the volume equal 5.00 L if the temperature is 58 °C?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (a) What mass of O2 does the tank contain?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (b) What volume would the gas occupy at STP?
