Treatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (f) What is the identity of the metal M?
Ch.13 - Solutions & Their Properties
Chapter 13, Problem 154
A solution prepared by dissolving 100.0 g of a mixture of sugar 1C12H22O112 and table salt (NaCl) in 500.0 g of water has a freezing point of - 2.25 °C. What is the mass of each individual solute? Assume that NaCl is completely dissociated.
 Verified step by step guidance
Verified step by step guidance1
Step 1: First, we need to understand the concept of freezing point depression. The freezing point of a solution is lower than that of the pure solvent, and this decrease in freezing point is directly proportional to the molality of the solution. The equation for this is ΔTf = Kf * m * i, where ΔTf is the freezing point depression, Kf is the cryoscopic constant of the solvent (for water, it's 1.86 °C/m), m is the molality of the solution, and i is the van't Hoff factor (the number of particles the solute splits into in solution). For sugar, i = 1 (since it doesn't dissociate), and for NaCl, i = 2 (since it dissociates into two ions).
Step 2: We know the total freezing point depression is -2.25 °C (the negative sign indicates a decrease in freezing point). We can set up two equations to represent the contributions of sugar and NaCl to the total freezing point depression: ΔTf,sugar = Kf * m_sugar and ΔTf,NaCl = Kf * m_NaCl * 2. Adding these two equations together gives us the total freezing point depression: ΔTf,total = ΔTf,sugar + ΔTf,NaCl = -2.25 °C.
Step 3: We also know that molality (m) is defined as the number of moles of solute per kilogram of solvent. We can express m_sugar as (mass_sugar / molar mass_sugar) / mass_solvent and m_NaCl as (mass_NaCl / molar mass_NaCl) / mass_solvent. Substituting these expressions into the equations from Step 2 gives us two equations with two unknowns (mass_sugar and mass_NaCl).
Step 4: We can solve these two equations simultaneously to find the values of mass_sugar and mass_NaCl. This can be done using various methods such as substitution, elimination, or using a system of equations solver.
Step 5: Once we have the values of mass_sugar and mass_NaCl, we can check our answers by making sure that they add up to the total mass of the solute (100.0 g), and that substituting them back into the equations from Step 2 gives us the correct total freezing point depression (-2.25 °C).

Verified Solution
Video duration:
16mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Freezing Point Depression
Freezing point depression is a colligative property that describes how the freezing point of a solvent decreases when a solute is added. The extent of this depression depends on the number of solute particles in the solution rather than their identity. The formula used to calculate the change in freezing point is ΔTf = i * Kf * m, where 'i' is the van 't Hoff factor, 'Kf' is the freezing point depression constant of the solvent, and 'm' is the molality of the solution.
Recommended video:
Guided course

Freezing Point Depression
Colligative Properties
Colligative properties are properties of solutions that depend on the number of solute particles in a given amount of solvent, not on the type of solute. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. Understanding these properties is essential for calculating how solutes affect the physical properties of solvents, which is crucial in this question.
Recommended video:
Guided course
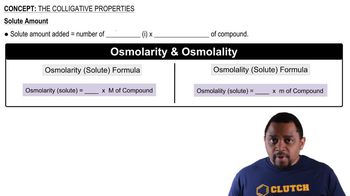
Colligative Properties
Dissociation of Ionic Compounds
Dissociation refers to the process by which an ionic compound separates into its constituent ions when dissolved in a solvent. For example, sodium chloride (NaCl) dissociates into Na+ and Cl- ions in solution. This dissociation increases the number of solute particles, which is important for calculating colligative properties like freezing point depression, as seen in the context of this question.
Recommended video:
Guided course

Ionic Compounds Naming
Related Practice
Textbook Question
411
views
Textbook Question
A compound that contains only C and H was burned in excess O2 to give CO2 and H2O. When 0.270 g of the com- pound was burned, the amount of CO2 formed reacted completely with 20.0 mL of 2.00 M NaOH solution according to the equation 2 OH-1aq2 + CO21g2 S CO 2- 1aq2 + H2O1l2 When 0.270 g of the compound was dissolved in 50.0 g of camphor, the resulting solution had a freezing point of 177.9 °C. [#Pure camphor freezes at 179.8 °C and has Kf = 37.7 1°C kg2>mol.] (a) What is the empirical formula of the compound?
616
views
Open Question
Combustion analysis of a 36.72-mg sample of the male hormone testosterone gave 106.43 mg CO2 and 32.10 mg H2O as the only combustion products. When 5.00 mg of testosterone was dissolved in 15.0 mL of a suitable solvent at 25 °C, an osmotic pressure of 21.5 mm Hg was measured. What is the molecular formula of testosterone?
