Here are the essential concepts you must grasp in order to answer the question correctly.
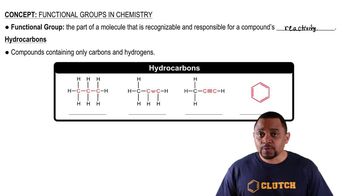
Hydrocarbons
Hydrocarbons are organic compounds composed solely of carbon (C) and hydrogen (H) atoms. They can be classified into two main categories: aliphatic hydrocarbons, which include alkanes, alkenes, and alkynes, and aromatic hydrocarbons, which contain one or more aromatic rings. Understanding the structure and types of hydrocarbons is essential for classifying organic compounds.
Recommended video:
Functionalized Hydrocarbons
Functionalized hydrocarbons are organic compounds that contain additional functional groups, which are specific groups of atoms that impart characteristic properties and reactivity to the molecule. These functional groups can include alcohols, amines, carboxylic acids, and more. Identifying these groups is crucial for classifying the compound and understanding its chemical behavior.
Recommended video:
Hydrocarbon Functional Groups
Alcohols
Alcohols are a family of functionalized hydrocarbons characterized by the presence of one or more hydroxyl (-OH) groups attached to a carbon atom. The compound in the question, H3C-CH2OH, is an alcohol known as ethanol, which belongs to the alcohol family. Recognizing the family of a functionalized hydrocarbon helps in predicting its properties and reactions.
Recommended video:
Rules for Naming Alcohols
 Verified step by step guidance
Verified step by step guidance