Here are the essential concepts you must grasp in order to answer the question correctly.
Quantum Numbers
Quantum numbers are a set of four numbers that describe the unique quantum state of an electron in an atom. They include the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). Each quantum number has specific rules governing its possible values, which are essential for determining the electron's energy level and orbital shape.
Recommended video:
Principal Quantum Number (n)
The principal quantum number (n) indicates the main energy level of an electron in an atom and can take positive integer values (1, 2, 3, ...). Higher values of n correspond to electrons that are further from the nucleus and have higher energy. In the given set, n = 2 is valid as it is a positive integer.
Recommended video:
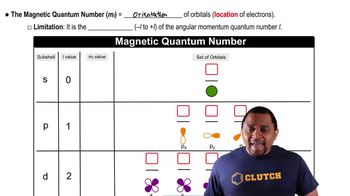
Azimuthal Quantum Number (l)
The azimuthal quantum number (l) defines the shape of the electron's orbital and can take integer values from 0 to (n-1). For n = 2, the possible values of l are 0 and 1, corresponding to the s and p orbitals, respectively. In the provided set, l = 2 is invalid because it exceeds the maximum allowed value for n = 2.
Recommended video: