Textbook Question
An energetically excited hydrogen atom has its electron in a 5f subshell. The electron drops down to the 3d subshell, releasing a photon in the process. (b) What wavelength of light is emitted by the process?
388
views
 Verified step by step guidance
Verified step by step guidance


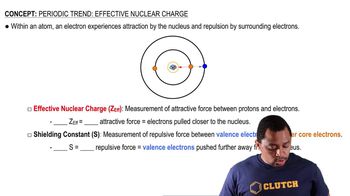
An energetically excited hydrogen atom has its electron in a 5f subshell. The electron drops down to the 3d subshell, releasing a photon in the process. (b) What wavelength of light is emitted by the process?
An energetically excited hydrogen atom has its electron in a 5f subshell. The electron drops down to the 3d subshell, releasing a photon in the process. (c) The hydrogen atom now has a single electron in the 3d subshell. What is the energy in kJ/mol required to remove this electron?
Consider the noble gas xenon. (a) Write the electron configuration of xenon using the abbreviation of the previous noble gas.