Here are the essential concepts you must grasp in order to answer the question correctly.
Crystal Field Theory
Crystal Field Theory (CFT) explains how the arrangement of ligands around a central metal ion affects the energy levels of the d-orbitals. In this theory, ligands create an electric field that splits the degenerate d-orbitals into different energy levels, influencing the color, magnetism, and stability of the complex.
Recommended video:
The study of ligand-metal interactions helped to form Ligand Field Theory which combines CFT with MO Theory.
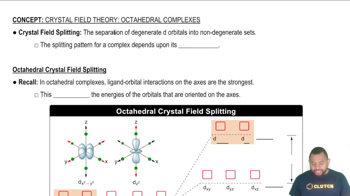
Tetrahedral vs. Octahedral Geometry
In tetrahedral geometry, four ligands are arranged around a central metal ion, leading to a specific splitting pattern of the d-orbitals. In contrast, octahedral geometry involves six ligands, resulting in a different splitting pattern. The arrangement of ligands directly affects the magnitude of crystal-field splitting energy (Δ).
Recommended video:
Crystal Field Splitting Energy (Δ)
Crystal Field Splitting Energy (Δ) is the energy difference between the split d-orbitals in a coordination complex. In general, Δ is larger in octahedral complexes compared to tetrahedral ones due to the stronger interaction between the ligands and the d-orbitals, which results from the greater number of ligands in octahedral geometry.
Recommended video:
The crystal field splitting pattern for octahedral complexes has the d orbitals on or along the axes as having the higher energy.
 Verified step by step guidance
Verified step by step guidance