Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. isopropyl alcohol (polar, contains an OH group) c. vegetable oil (nonpolar) d. sodium nitrate (ionic)
Ch.13 - Solutions
Chapter 13, Problem 32
Which molecule would you expect to be more soluble in water: CCl4 or CH2Cl2?
 Verified step by step guidance
Verified step by step guidance1
Identify the key property that affects solubility in water: polarity. Water is a polar solvent, so polar molecules are generally more soluble in water than nonpolar molecules.
Examine the molecular structure of CCl4 (carbon tetrachloride). It is a symmetrical molecule with four chlorine atoms bonded to a central carbon atom. This symmetry makes CCl4 a nonpolar molecule.
Examine the molecular structure of CH2Cl2 (dichloromethane). It has two hydrogen atoms and two chlorine atoms bonded to a central carbon atom. The presence of the more electronegative chlorine atoms creates a dipole moment, making CH2Cl2 a polar molecule.
Compare the polarity of the two molecules: CCl4 is nonpolar, while CH2Cl2 is polar. Since water is a polar solvent, it will better dissolve polar substances.
Conclude that CH2Cl2, being polar, is expected to be more soluble in water than CCl4, which is nonpolar.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity
Polarity refers to the distribution of electrical charge over the atoms in a molecule. Polar molecules have a significant difference in electronegativity between their atoms, leading to a dipole moment. This characteristic affects solubility, as polar substances tend to dissolve well in polar solvents like water, while nonpolar substances do not.
Recommended video:
Guided course

Molecular Polarity
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). In the context of solubility, stronger intermolecular forces between solute and solvent molecules enhance solubility. Water, being a polar solvent, interacts favorably with polar solutes through hydrogen bonding and dipole-dipole interactions.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Like Dissolves Like
The principle of 'like dissolves like' states that polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. This concept is crucial for predicting solubility; since water is polar, it will more readily dissolve polar molecules, such as CH2Cl2, compared to nonpolar molecules like CCl4.
Recommended video:
Guided course
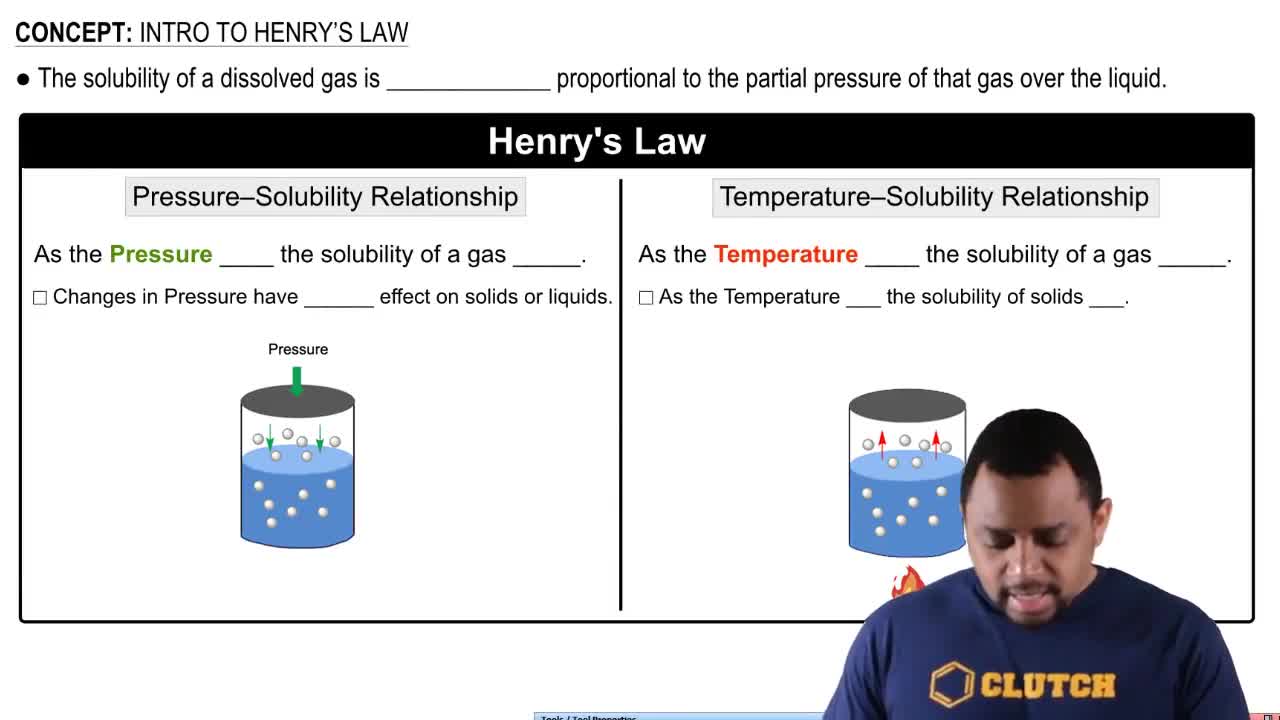
Henry's Law Solubility
Related Practice
Textbook Question
Textbook Question
Pick an appropriate solvent from Table 13.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. b. sodium chloride (ionic)
867
views
Textbook Question
Which molecule would you expect to be more soluble in water: CH3CH2CH2OH or HOCH2CH2CH2OH?
3970
views
Textbook Question
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most soluble. a. glucose
598
views
Textbook Question
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble. d. ethylene glycol
712
views
Open Question
When ammonium chloride (NH4Cl) is dissolved in water, the solution becomes colder: a. Is the dissolution of ammonium chloride endothermic or exothermic? b. What can you conclude about the relative magnitudes of the lattice energy of ammonium chloride and its heat of hydration? c. Sketch a qualitative energy diagram similar to Figure 13.7 for the dissolution of NH4Cl. d. Why does the solution form? What drives the process?
