Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2(s) + 5 F2(g) → 2 IF5(g) A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (a) What is the partial pressure of IF5 in the flask?
Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2(s) + 5 F2(g) → 2 IF5(g) A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (d) What is the total mass of reactants and products in the flask?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Law of Conservation of Mass
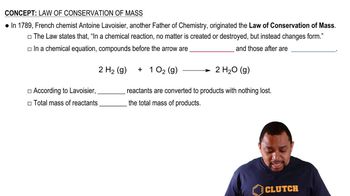
Stoichiometry

Molar Mass

Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2(s) + 5 F2(g) → 2 IF5(g) A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (b) What is the mole fraction of IF5 in the flask?
Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2(s) + 5 F2(g) → 2 IF5(g) A 5.00-L flask containing 10.0 g of I2 is charged with 10.0 g of F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 °C. (c) Draw the Lewis structure of IF5.
A 6.53-g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas at 28 °C and 99.06 kPa pressure. (a) Write balanced chemical equations for the reactions that occur between hydrochloric acid and each component of the mixture.
A 6.53-g sample of a mixture of magnesium carbonate and calcium carbonate is treated with excess hydrochloric acid. The resulting reaction produces 1.72 L of carbon dioxide gas at 28 °C and 99.06 kPa pressure. (c) Assuming that the reactions are complete, calculate the percentage by mass of magnesium carbonate in the mixture.
