Textbook Question
How many protons and neutrons are in the nucleus of the following atoms? (b)
283
views

 Verified step by step guidance
Verified step by step guidance

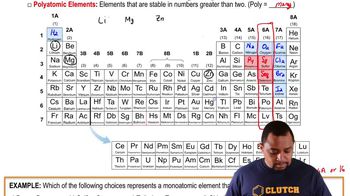

How many protons and neutrons are in the nucleus of the following atoms? (b)
How many protons and neutrons are in the nucleus of the following atoms? (c)
How many protons and neutrons are in the nucleus of the following atoms? (d)
The molar mass of HCl is 36.5 g/mol, and the average mass per HCl molecule is 36.5 u. Use the fact that 1 u = 1.6605 * 102 24g to calculate Avogadro's number.