Olympic cyclists fill their tires with helium to make them lighter. Calculate the mass of air in an air-filled tire and the mass of helium in a helium-filled tire. Assume that the volume of the tire is 855 mL, that it is filled to a total pressure of 125 psi, and that the temperature is 25 °C. Also, assume an average molar mass for air of 28.8 g/mol. Calculate the mass of air in an air-filled tire.
Gaseous ammonia is injected into the exhaust stream of a coal-burning power plant to reduce the pollutant NO to N2 according to the reaction: 4 NH3(g) + 4 NO(g) + O2(g) → 4 N2(g) + 6 H2O(g). Suppose that the exhaust stream of a power plant has a flow rate of 335 L/s at a temperature of 955 K, and that the exhaust contains a partial pressure of NO of 22.4 torr. What should be the flow rate of ammonia delivered at 755 torr and 298 K into the stream to react completely with the NO if the ammonia is 65.2% pure (by volume)?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Stoichiometry

Ideal Gas Law

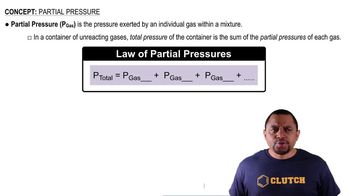
Partial Pressure and Gas Mixtures
Olympic cyclists fill their tires with helium to make them lighter. Calculate the mass of air in an air-filled tire and the mass of helium in a helium-filled tire. Assume that the volume of the tire is 855 mL, that it is filled to a total pressure of 125 psi, and that the temperature is 25 °C. Also, assume an average molar mass for air of 28.8 g/mol. Calculate the mass of helium in a helium-filled tire.
Olympic cyclists fill their tires with helium to make them lighter. Calculate the mass of air in an air-filled tire and the mass of helium in a helium-filled tire. Assume that the volume of the tire is 855 mL, that it is filled to a total pressure of 125 psi, and that the temperature is 25 °C. Also, assume an average molar mass for air of 28.8 g/mol. What is the mass difference between the two?
An ordinary gasoline can measuring 30.0 cm by 20.0 cm by 15.0 cm is evacuated with a vacuum pump. Assuming that virtually all of the air can be removed from inside the can and that atmospheric pressure is 14.7 psi, what is the total force (in pounds) on the surface of the can? Do you think that the can could withstand the force?
