Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that measures the maximum reversible work obtainable from a thermodynamic system at constant temperature and pressure. It is crucial for determining the spontaneity of a reaction; a negative change in Gibbs Free Energy (ΔG) indicates that a reaction can occur spontaneously. The relationship between Gibbs Free Energy and cell potential is given by the equation ΔG = -nFE, where n is the number of moles of electrons transferred, F is Faraday's constant, and E is the cell potential.
Recommended video:
Gibbs Free Energy of Reactions
Cell Potential
Cell potential (E) is the measure of the voltage produced by an electrochemical cell during a redox reaction. It reflects the tendency of the cell to drive the reaction forward; a higher cell potential indicates a greater likelihood of the reaction occurring spontaneously. In the context of the silver oxide-zinc battery, the given voltage of 1.60 V is essential for calculating the free-energy change using the Gibbs Free Energy equation.
Recommended video:
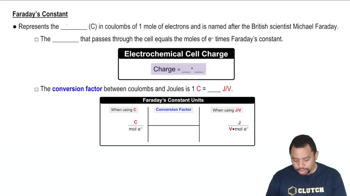
Faraday's Constant
Faraday's Constant (F) is the amount of electric charge per mole of electrons, approximately 96485 C/mol. It is a key value in electrochemistry, linking the amount of substance involved in a reaction to the electric charge required to drive that reaction. In calculations involving electrochemical cells, Faraday's Constant is used to convert the cell potential and the number of moles of electrons into Gibbs Free Energy, allowing for the determination of the energy change associated with the cell reaction.
Recommended video:
Faraday's Constant in Electrochemistry