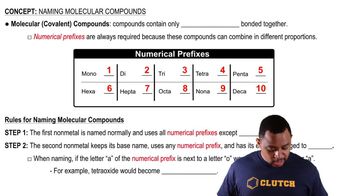
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Compounds
Molecular compounds are formed when two or more nonmetals bond together by sharing electrons, resulting in covalent bonds. These compounds typically have low melting and boiling points and exist as gases or liquids at room temperature. An example is N2O4, which consists of nitrogen and oxygen atoms bonded covalently.
Recommended video:
Naming Molecular Compounds
Ionic Compounds
Ionic compounds are formed when metals transfer electrons to nonmetals, resulting in the formation of charged ions that attract each other due to electrostatic forces. These compounds usually have high melting and boiling points and are often soluble in water. A common example is sodium chloride (NaCl), formed from sodium and chlorine.
Recommended video:
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. In general, a large difference in electronegativity between two atoms indicates an ionic bond, while a small difference suggests a covalent bond. Understanding electronegativity helps predict the nature of the bond in compounds like N2O4, which is covalent due to the similar electronegativities of nitrogen and oxygen.
Recommended video:
 Verified step by step guidance
Verified step by step guidance