Here are the essential concepts you must grasp in order to answer the question correctly.
Isomers
Isomers are compounds that have the same molecular formula but differ in the arrangement of atoms. This can lead to different physical and chemical properties. There are two main types of isomers: structural isomers, which differ in the connectivity of their atoms, and stereoisomers, which have the same connectivity but differ in the spatial arrangement of atoms.
Recommended video:
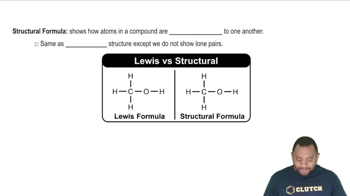
Structural Formula
A structural formula represents the arrangement of atoms within a molecule, showing how the atoms are bonded to each other. It provides insight into the connectivity and functional groups present in the molecule, which is crucial for determining whether two structures are identical or different. Understanding structural formulas helps in visualizing isomerism.
Recommended video:
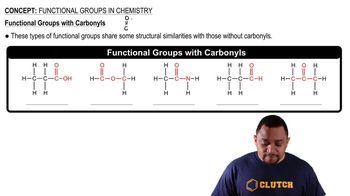
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Identifying functional groups is essential in distinguishing between isomers, as different arrangements can lead to different functional groups, affecting the molecule's reactivity and properties.
Recommended video:
Carbonyl Functional Groups

 Verified step by step guidance
Verified step by step guidance