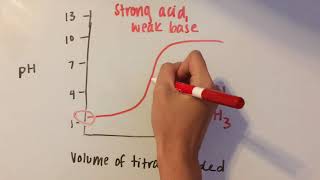
SPEAKER: So in this experiment, we're going to learn about the principles and procedures of a basic acid base titration. So in this experiment, we're going to titrate acetic acid with sodium hydroxide. And the products are gonna be acetate ion and water. So generally what we want is we know the concentration of our sodium hydroxide. But the concentration of our acetic acid solution is unknown. So we want to determine this concentration. And we do that with a titration. Here's the basic equipment that we're using in the titration. We have a volumetric burette. You notice all the little lines on here? These are used for measuring the volume. Generally we can measure the volume to about 0.02 milliliters. And in here, we have a beaker containing our acetic acid. We call the acetic acid, the analyte. And in the burette, we have the sodium hydroxide. We call that the titrant. We have added 25.00 milliliters of acetic acid using this pipette. Pipette is used for delivering accurate volumes of fixed volumes. In this case, this is a 25 milliliter pipette. So we're going to titrate, or we're going to deliver the sodium hydroxide into the acetic acid and consume the acetic acid. And when we're done, we will measure the volume of the sodium hydroxide required. And then from that, we can calculate the concentration. But the question is, how do we know when we're done? How do we know when we've added just enough sodium hydroxide to consume all of these acetic acid but we haven't added too much? So if we go back here to the board, I've drawn here a graph, a rough graph of what a pH will look like as a function of the volume of the titrant, or in this case, the volume of the sodium hydroxide. In other words, this is what the pH is going to do as we add sodium hydroxide. So as you can see, the pH is low because acetic acid is an acid. And then as we add sodium hydroxide, our pH will come down on to be a plateau region. We call this the buffer region. And then it's gonna rise quite steeply. Right here in the middle, that is when we have exactly consumed all of the acetic acid. But how do we know when we get there? Notice here that the sodium hydroxide is colorless. The acetic acid is colorless. And the products are colorless. So how do we know when we do that? Well, in this case, we're gonna use an indicator. This is phenolphthalein. And we're gonna add three drops. And what that's gonna do is that's gonna change color. So phenolphthalein is gonna be colorless when it's acidic. And as it gets more basic, at some point, it's gonna change color to pink. And when it changes color to pink, we're gonna stop. And we're going to assume that that point is when we've consumed all the acetic acid. We call that the endpoint. If my burette contains approximately 0.1 molar sodium hydroxide and my acetic acid is also approximately 0.1 molar in concentration, although we don't know exactly what it is, and we've added 25 milliliters of this acetic acid, what would you think would be the volume, the approximate volume of sodium hydroxide we will add to reach that endpoint? So if the sodium hydroxide is approximately 0.1 molar and the acetic acid is approximately 0.1 molar and we've added 25 milliliters of that, then the volume of the sodium hydroxide that we will need to add, it's also approximately 25 milliliters. But that's approximate, so we need to be careful. So we're going to do our titration now. We've added our phenolphthalein failing indicator. And we've zeroed out our volume. We have one meniscus here that measures-- that's zeroed out on our burette. And now we're gonna do our titration. We have here our stop cork that we can use to control the flow. And we're going to go quickly down to about 22 milliliters and then go slowly until we get that pink color. So here we'll start. And so we're at 2 milliliters. And it'll just take a little while to get down. So we are at 7 milliliters, 12 milliliters, down to about 19. And I'm gonna start slowing down a little bit. Down to 20. Notice that I'm adding dropwise. 21 milliliters. And now at 22 milliliters. Still colorless, but we're close. So we wanna be careful here. Each drop that we add is 0.07 milliliters in volume. But remember that we can measure 0.02 milliliters on the volume of a burette. So we need to add fractions of drops. And we do that by suspending drops and squirting a little bit of distilled water into it. So we're gonna go add a drop at a Be very careful here. So there is a drop. Notice I have on the end of it, a little drop suspended. And I'm gonna to squirt that off with my water bottle. And I'm gonna no longer worry about my volume and just look at my color. Another drop. And you're looking for a little-- when a drop enters, how long does it take for the pink to go away? So here's another 1/2 a drop I've suspended. I'm gonna squirt it off. And I'm noticing that I'm getting a little bit of pink when those sodium hydroxide goes in. There's a drop suspended. Squirt that one off. You can tell by how long the pink lasts before it disappears. Patience is what's important here. Most students make mistakes because they lose their patience. And pink disappeared. We probably have half a drop to go. And there we go. It's pink. It's pinks placid. So the volume of titrant added or the volume of the sodium hydroxide was 23.05 milliliters. Let's go put that on the board. And let's go do a calculation. So here, volume of the sodium hydroxide was 23.05 milliliters. Up here, remember concentration of the sodium hydroxide, 0.1002, this is known. Volume of the acetic acid we added was 25 milliliters, volume of the titrant, 23.05 milliliters. What would be the concentration of the unknown acetic acid? Let's go do the calculation. So we started with 23.05 milliliters of sodium hydroxide. Now, the concentration of that was 0.1002 millimole per milliliter of sodium hydroxide. Now, we're using millimoles. Might be unfamiliar. But it's millimoles per milliliters concentration and is often moles per liter. But millimoles per milliliter gives us the same thing. And this is more convenient because the milliliters of sodium hydroxide cancel the milliliters of sodium hydroxide here, giving us millimoles of sodium hydroxide. Now, from our reaction that we indicated previously, there was one millimole of acetic acid per one millimole of sodium hydroxide, canceling our millimoles of sodium hydroxide, giving us millimoles of acetic acid. But we want concentration. Concentration is moles per liter or millimoles per milliliter. So we're going to divide here by our volume of acetic acid, which is 25.00 milliliters of acetic acid, giving us millimoles per milliliter. And this will give us our answer, 0.0924 molar acetic acid.
Table of contents
- 1. Intro to General Chemistry3h 46m
- Classification of Matter16m
- Physical & Chemical Changes19m
- Chemical Properties7m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature12m
- Scientific Notation13m
- SI Units7m
- Metric Prefixes24m
- Significant Figures9m
- Significant Figures: Precision in Measurements8m
- Significant Figures: In Calculations14m
- Conversion Factors16m
- Dimensional Analysis17m
- Density12m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects7m
- 2. Atoms & Elements4h 16m
- The Atom9m
- Subatomic Particles15m
- Isotopes17m
- Ions27m
- Atomic Mass28m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Element Symbols6m
- Periodic Table: Elemental Forms6m
- Periodic Table: Phases8m
- Periodic Table: Charges20m
- Calculating Molar Mass10m
- Mole Concept30m
- Law of Conservation of Mass5m
- Law of Definite Proportions10m
- Atomic Theory9m
- Law of Multiple Proportions3m
- Millikan Oil Drop Experiment7m
- Rutherford Gold Foil Experiment10m
- 3. Chemical Reactions4h 10m
- Empirical Formula18m
- Molecular Formula20m
- Combustion Analysis38m
- Combustion Apparatus15m
- Polyatomic Ions24m
- Naming Ionic Compounds11m
- Writing Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- Naming Molecular Compounds6m
- Balancing Chemical Equations13m
- Stoichiometry16m
- Limiting Reagent17m
- Percent Yield19m
- Mass Percent4m
- Functional Groups in Chemistry11m
- 4. BONUS: Lab Techniques and Procedures1h 38m
- 5. BONUS: Mathematical Operations and Functions48m
- 6. Chemical Quantities & Aqueous Reactions3h 51m
- Solutions6m
- Molarity18m
- Osmolarity15m
- Dilutions12m
- Solubility Rules16m
- Electrolytes18m
- Molecular Equations18m
- Gas Evolution Equations13m
- Solution Stoichiometry14m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Activity Series10m
- 7. Gases3h 52m
- Pressure Units6m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws13m
- Chemistry Gas Laws: Combined Gas Law12m
- Mole Fraction of Gases6m
- Partial Pressure19m
- The Ideal Gas Law: Molar Mass16m
- The Ideal Gas Law: Density14m
- Gas Stoichiometry18m
- Standard Temperature and Pressure14m
- Effusion13m
- Root Mean Square Speed9m
- Kinetic Energy of Gases10m
- Maxwell-Boltzmann Distribution8m
- Velocity Distributions4m
- Kinetic Molecular Theory14m
- Van der Waals Equation9m
- 8. Thermochemistry2h 37m
- Nature of Energy6m
- Kinetic & Potential Energy9m
- First Law of Thermodynamics7m
- Internal Energy8m
- Endothermic & Exothermic Reactions7m
- Heat Capacity19m
- Constant-Pressure Calorimetry22m
- Constant-Volume Calorimetry10m
- Thermal Equilibrium8m
- Thermochemical Equations12m
- Formation Equations9m
- Enthalpy of Formation12m
- Hess's Law23m
- 9. Quantum Mechanics2h 58m
- Wavelength and Frequency6m
- Speed of Light8m
- The Energy of Light13m
- Electromagnetic Spectrum10m
- Photoelectric Effect17m
- De Broglie Wavelength9m
- Heisenberg Uncertainty Principle17m
- Bohr Model14m
- Emission Spectrum5m
- Bohr Equation13m
- Introduction to Quantum Mechanics5m
- Quantum Numbers: Principal Quantum Number5m
- Quantum Numbers: Angular Momentum Quantum Number10m
- Quantum Numbers: Magnetic Quantum Number11m
- Quantum Numbers: Spin Quantum Number9m
- Quantum Numbers: Number of Electrons11m
- Quantum Numbers: Nodes6m
- 10. Periodic Properties of the Elements3h 10m
- The Electron Configuration22m
- The Electron Configuration: Condensed4m
- The Electron Configurations: Exceptions13m
- The Electron Configuration: Ions12m
- Paramagnetism and Diamagnetism9m
- The Electron Configuration: Quantum Numbers16m
- Valence Electrons of Elements12m
- Periodic Trend: Metallic Character3m
- Periodic Trend: Atomic Radius8m
- Periodic Trend: Ionic Radius13m
- Periodic Trend: Ionization Energy12m
- Periodic Trend: Successive Ionization Energies11m
- Periodic Trend: Electron Affinity10m
- Periodic Trend: Electronegativity5m
- Periodic Trend: Effective Nuclear Charge21m
- Periodic Trend: Cumulative12m
- 11. Bonding & Molecular Structure3h 29m
- Lewis Dot Symbols10m
- Chemical Bonds13m
- Dipole Moment11m
- Octet Rule10m
- Formal Charge9m
- Lewis Dot Structures: Neutral Compounds20m
- Lewis Dot Structures: Sigma & Pi Bonds14m
- Lewis Dot Structures: Ions15m
- Lewis Dot Structures: Exceptions14m
- Lewis Dot Structures: Acids15m
- Resonance Structures21m
- Average Bond Order4m
- Bond Energy15m
- Coulomb's Law6m
- Lattice Energy12m
- Born Haber Cycle14m
- 12. Molecular Shapes & Valence Bond Theory1h 57m
- 13. Liquids, Solids & Intermolecular Forces2h 23m
- Molecular Polarity10m
- Intermolecular Forces20m
- Intermolecular Forces and Physical Properties11m
- Clausius-Clapeyron Equation18m
- Phase Diagrams13m
- Heating and Cooling Curves27m
- Atomic, Ionic, and Molecular Solids11m
- Crystalline Solids4m
- Simple Cubic Unit Cell7m
- Body Centered Cubic Unit Cell12m
- Face Centered Cubic Unit Cell6m
- 14. Solutions3h 1m
- Solutions: Solubility and Intermolecular Forces17m
- Molality15m
- Parts per Million (ppm)13m
- Mole Fraction of Solutions8m
- Solutions: Mass Percent12m
- Types of Aqueous Solutions8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- The Colligative Properties14m
- Boiling Point Elevation16m
- Freezing Point Depression10m
- Osmosis19m
- Osmotic Pressure10m
- Vapor Pressure Lowering (Raoult's Law)16m
- 15. Chemical Kinetics2h 53m
- 16. Chemical Equilibrium2h 29m
- 17. Acid and Base Equilibrium5h 1m
- Acids Introduction9m
- Bases Introduction7m
- Binary Acids15m
- Oxyacids10m
- Bases14m
- Amphoteric Species5m
- Arrhenius Acids and Bases5m
- Bronsted-Lowry Acids and Bases21m
- Lewis Acids and Bases12m
- The pH Scale16m
- Auto-Ionization9m
- Ka and Kb16m
- pH of Strong Acids and Bases9m
- Ionic Salts17m
- pH of Weak Acids31m
- pH of Weak Bases32m
- Diprotic Acids and Bases8m
- Diprotic Acids and Bases Calculations30m
- Triprotic Acids and Bases9m
- Triprotic Acids and Bases Calculations17m
- 18. Aqueous Equilibrium4h 47m
- Intro to Buffers20m
- Henderson-Hasselbalch Equation19m
- Intro to Acid-Base Titration Curves13m
- Strong Titrate-Strong Titrant Curves9m
- Weak Titrate-Strong Titrant Curves15m
- Acid-Base Indicators8m
- Titrations: Weak Acid-Strong Base38m
- Titrations: Weak Base-Strong Acid41m
- Titrations: Strong Acid-Strong Base11m
- Titrations: Diprotic & Polyprotic Buffers32m
- Solubility Product Constant: Ksp17m
- Ksp: Common Ion Effect18m
- Precipitation: Ksp vs Q12m
- Selective Precipitation9m
- Complex Ions: Formation Constant18m
- 19. Chemical Thermodynamics1h 50m
- 20. Electrochemistry2h 42m
- 21. Nuclear Chemistry2h 34m
- Intro to Radioactivity10m
- Alpha Decay9m
- Beta Decay7m
- Gamma Emission7m
- Electron Capture & Positron Emission8m
- Neutron to Proton Ratio7m
- Band of Stability: Alpha Decay & Nuclear Fission10m
- Band of Stability: Beta Decay3m
- Band of Stability: Electron Capture & Positron Emission4m
- Band of Stability: Overview14m
- Measuring Radioactivity7m
- Rate of Radioactive Decay12m
- Radioactive Half-Life16m
- Mass Defect18m
- Nuclear Binding Energy14m
- 22. Organic Chemistry5h 7m
- Introduction to Organic Chemistry8m
- Structural Formula8m
- Condensed Formula10m
- Skeletal Formula6m
- Spatial Orientation of Bonds3m
- Intro to Hydrocarbons16m
- Isomers11m
- Chirality15m
- Functional Groups in Chemistry11m
- Naming Alkanes4m
- The Alkyl Groups9m
- Naming Alkanes with Substituents13m
- Naming Cyclic Alkanes6m
- Naming Other Substituents8m
- Naming Alcohols11m
- Naming Alkenes11m
- Naming Alkynes9m
- Naming Ketones5m
- Naming Aldehydes5m
- Naming Carboxylic Acids4m
- Naming Esters8m
- Naming Ethers5m
- Naming Amines5m
- Naming Benzene7m
- Alkane Reactions7m
- Intro to Addition Reactions4m
- Halogenation Reactions4m
- Hydrogenation Reactions3m
- Hydrohalogenation Reactions7m
- Alcohol Reactions: Substitution Reactions4m
- Alcohol Reactions: Dehydration Reactions9m
- Intro to Redox Reactions8m
- Alcohol Reactions: Oxidation Reactions7m
- Aldehydes and Ketones Reactions6m
- Ester Reactions: Esterification4m
- Ester Reactions: Saponification3m
- Carboxylic Acid Reactions4m
- Amine Reactions3m
- Amide Formation4m
- Benzene Reactions10m
- 23. Chemistry of the Nonmetals2h 39m
- Main Group Elements: Bonding Types4m
- Main Group Elements: Boiling & Melting Points7m
- Main Group Elements: Density11m
- Main Group Elements: Periodic Trends7m
- The Electron Configuration Review16m
- Periodic Table Charges Review20m
- Hydrogen Isotopes4m
- Hydrogen Compounds11m
- Production of Hydrogen8m
- Group 1A and 2A Reactions7m
- Boron Family Reactions7m
- Boron Family: Borane7m
- Borane Reactions7m
- Nitrogen Family Reactions12m
- Oxides, Peroxides, and Superoxides12m
- Oxide Reactions4m
- Peroxide and Superoxide Reactions6m
- Noble Gas Compounds3m
- 24. Transition Metals and Coordination Compounds3h 16m
- Atomic Radius & Density of Transition Metals11m
- Electron Configurations of Transition Metals7m
- Electron Configurations of Transition Metals: Exceptions11m
- Paramagnetism and Diamagnetism10m
- Ligands10m
- Complex Ions5m
- Coordination Complexes7m
- Classification of Ligands11m
- Coordination Numbers & Geometry9m
- Naming Coordination Compounds22m
- Writing Formulas of Coordination Compounds8m
- Isomerism in Coordination Complexes14m
- Orientations of D Orbitals4m
- Intro to Crystal Field Theory10m
- Crystal Field Theory: Octahedral Complexes5m
- Crystal Field Theory: Tetrahedral Complexes4m
- Crystal Field Theory: Square Planar Complexes4m
- Crystal Field Theory Summary8m
- Magnetic Properties of Complex Ions9m
- Strong-Field vs Weak-Field Ligands6m
- Magnetic Properties of Complex Ions: Octahedral Complexes11m
18. Aqueous Equilibrium
Intro to Acid-Base Titration Curves
Video duration:
9mPlay a video:
Related Videos
Related Practice