Here are the essential concepts you must grasp in order to answer the question correctly.
Henry's Law
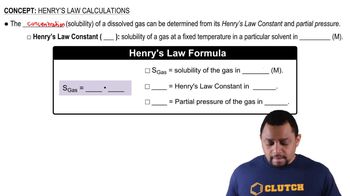
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This relationship can be expressed mathematically as C = kH * P, where C is the concentration of the dissolved gas, kH is the Henry's Law constant, and P is the partial pressure of the gas.
Recommended video:
Molar Solubility
Molar solubility refers to the maximum amount of a solute that can dissolve in a given volume of solvent at a specific temperature, expressed in moles per liter (mol/L). It is a crucial concept in understanding how gases behave in solutions, particularly under varying pressure conditions as described by Henry's Law.
Recommended video:
Temperature Effects on Solubility
Temperature significantly influences the solubility of gases in liquids. Generally, as temperature increases, the solubility of gases decreases due to increased kinetic energy, which allows gas molecules to escape from the liquid phase. Understanding this relationship is essential when applying Henry's Law to calculate solubility at different temperatures.
Recommended video:
 Verified step by step guidance
Verified step by step guidance