Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Character
Covalent character refers to the extent to which a bond between two atoms exhibits characteristics of covalent bonding, as opposed to ionic bonding. It is influenced by factors such as the electronegativity difference between the bonded atoms and the size of the ions involved. Generally, smaller and more electronegative atoms tend to form bonds with higher covalent character.
Recommended video:
Metallic Character Example
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. The greater the difference in electronegativity between two atoms, the more ionic the bond is likely to be. In contrast, bonds between atoms with similar electronegativities tend to be more covalent, which is crucial for determining the covalent character of oxides.
Recommended video:
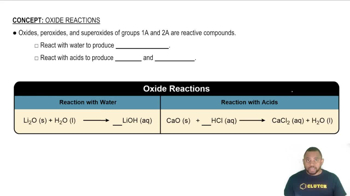
Oxide Types
Oxides can be classified based on their bonding characteristics and the elements involved. For example, metal oxides like BeO and Li2O typically exhibit ionic character, while non-metal oxides like CO2 and N2O5 show more covalent character. Understanding the nature of the oxides in question helps in arranging them according to their increasing covalent character.
Recommended video:
 Verified step by step guidance
Verified step by step guidance