Using Heisenberg’s uncertainty principle, calculate the uncertainty in the position of b. a proton moving at a speed of (5.00±0.01) × 104 m/s. The mass of a proton is 1.673×10−27 kg.
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 56b
How many unique combinations of the quantum numbers l and 𝑚𝑙 are there when b. n = 4?
 Verified step by step guidance
Verified step by step guidance1
<em>n</em> is the principal quantum number, and it determines the energy level of an electron in an atom. For <em>n</em> = 4, the possible values of the azimuthal quantum number <em>l</em> range from 0 to <em>n</em> - 1.
For each value of <em>l</em>, the magnetic quantum number <em>m<sub>l</sub></em> can take integer values from -<em>l</em> to +<em>l</em>, including zero.
Calculate the number of possible <em>m<sub>l</sub></em> values for each <em>l</em> value. For example, if <em>l</em> = 0, <em>m<sub>l</sub></em> can only be 0, giving 1 combination.
Continue this process for <em>l</em> = 1, 2, and 3, calculating the number of <em>m<sub>l</sub></em> values for each.
Sum the number of combinations for each <em>l</em> value to find the total number of unique combinations of <em>l</em> and <em>m<sub>l</sub></em> when <em>n</em> = 4.

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Quantum Numbers
Quantum numbers are sets of numerical values that describe the unique quantum state of an electron in an atom. They include the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). Each quantum number provides specific information about the electron's energy level, shape of the orbital, orientation, and spin.
Recommended video:
Guided course

Principal Quantum Number
Azimuthal Quantum Number (l)
The azimuthal quantum number (l) determines the shape of an electron's orbital and can take on integer values from 0 to n-1, where n is the principal quantum number. For n = 4, l can be 0, 1, 2, or 3, corresponding to the s, p, d, and f orbitals, respectively. Each value of l defines a different type of orbital with distinct shapes.
Recommended video:
Guided course
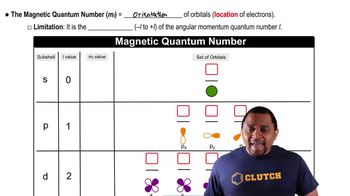
Magnetic Quantum Number
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) specifies the orientation of an orbital in space and can take on integer values ranging from -l to +l, including zero. For each value of l, there are 2l + 1 possible values of ml. For example, if l = 2 (d orbital), ml can be -2, -1, 0, +1, or +2, resulting in five unique orientations.
Recommended video:
Guided course

Magnetic Quantum Number
Related Practice
Textbook Question
1
views
Textbook Question
Calculate the uncertainty in the position of (a) an electron moving at a speed of 13.00 ± 0.012 × 105 m/s (b) a neutron moving at this same speed. (The masses of an electron and a neutron are given in the table of fundamental constants in the inside cover of the text.)
900
views
1
rank
Textbook Question
(a) For n = 4, what are the possible values of l?
1164
views
Textbook Question
Give the numerical values of n and l corresponding to each of the following orbital designations: (a) 3p (b) 2s (c) 4f
505
views
Textbook Question
Give the numerical values of n and l corresponding to each of the following orbital designations: (d) 5d.
791
views
Textbook Question
Give the values for n, l, and 𝑚𝑙 for a. each orbital in the 2p subshell
2
views
