Write equations that show the process for a. the first two ionization energies of lead and b. the fourth ionization energy of zirconium.
Pick the correct word to complete the sentence: The larger the atom, the (smaller/larger) its first ionization energy.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Ionization Energy

Atomic Size

Trends in Ionization Energy
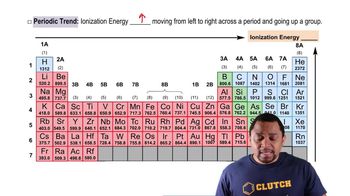
Which element has the highest second ionization energy: Li, K, or Be?
Identify each statement as true or false.
a. Ionization energies are always negative quantities.
b. Oxygen has a larger first ionization energy than fluorine.
c. The second ionization energy of an atom is always greater than its first ionization energy.
d. The third ionization energy is the energy needed to ionize three electrons from a neutral atom.
Which element in the periodic table has the largest first ionization energy?
Would a neutral K atom or a K+ ion have a more negative value of electron affinity?
Consider the following equation:
Ca+(𝑔)+e−⟶Ca(𝑔)
Which of the following statements are true?
i. The energy change for this process is the electron affinity of the Ca+ ion.
ii. The energy change for this process is the negative of the first ionization energy of the Ca atom.
iii. The energy change for this process is the negative of the electron affinity of the Ca atom.
a. Only statement i is true.
b. Only statement ii is true.
c. Only statement iii is true.
d. Only statements i and ii are true.
e. All three statements are true.
