Here are the essential concepts you must grasp in order to answer the question correctly.
Colligative Properties
Colligative properties depend on the number of solute particles in a solution rather than their identity. In the context of seawater, the presence of NaCl and MgCl2 contributes to the total solute concentration, affecting properties like vapor pressure, boiling point, and osmotic pressure. Understanding these properties is essential for calculating the osmotic pressure exerted by the seawater, which influences the efficiency of reverse osmosis.
Recommended video:
Osmotic Pressure
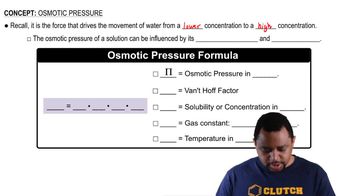
Osmotic pressure is the pressure required to stop the flow of solvent into a solution through a semipermeable membrane. It can be calculated using the formula π = iCRT, where π is the osmotic pressure, i is the van 't Hoff factor (number of particles the solute dissociates into), C is the molarity of the solution, R is the ideal gas constant, and T is the temperature in Kelvin. This concept is crucial for determining the maximum pressure needed to reverse the natural osmotic flow of water from seawater to freshwater.
Recommended video:
Reverse Osmosis
Reverse osmosis is a water purification process that uses a semipermeable membrane to remove ions, molecules, and larger particles from drinking water. By applying pressure greater than the osmotic pressure of the solution, water is forced through the membrane, leaving contaminants behind. Understanding the principles of reverse osmosis is vital for calculating how much freshwater can be produced from seawater under specific pressure conditions.
Recommended video: