Label each of the following substances as an acid, base, salt, or none of the above. Indicate whether the substance exists in aqueous solution entirely in molecular form, entirely as ions, or as a mixture of molecules and ions. (c) NaClO4
Classify each of the following substances as a nonelectrolyte, weak electrolyte, or strong electrolyte in water: a. H2SO3
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Electrolytes

Strong vs. Weak Electrolytes

Sulfurous Acid (H2SO3)
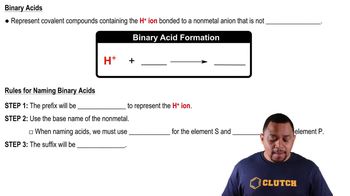
Label each of the following substances as an acid, base, salt, or none of the above. Indicate whether the substance exists in aqueous solution entirely in molecular form, entirely as ions, or as a mixture of molecules and ions. (d) Ba1OH22.
An aqueous solution of an unknown solute is tested with litmus paper and found to be acidic. The solution is weakly conducting compared with a solution of NaCl of the same concentration. Which of the following substances could the unknown be: KOH, NH3, HNO3, KClO2, H3PO3, CH3COCH3 (acetone)?
Complete and balance the following molecular equations, and then write the net ionic equation for each:
(a) HBr(aq) + Ca(OH)2(aq) →
(b) Cu(OH)2(s) + HClO4(aq) →
(c) Al(OH)3(s) + HNO3(aq) →
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (a) Aqueous acetic acid is neutralized by aqueous barium hydroxide
Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (b) Solid chromium(III) hydroxide reacts with nitrous acid.
