The dissolution of CaCl2(s) in water is exothermic, with ΔHsoln= - 81.3 kJ>mol. If you were to prepare a 1.00 m solution of CaCl2 beginning with water at 25.0 °C, what would the final temperature of the solution be in °C? Assume that the specific heats of both pure H2O and the solution are the same, 4.18 J>1K g2.
Ch.13 - Solutions & Their Properties
Chapter 13, Problem 59a
Residues of the herbicide atrazine (C8H14ClN5) in water can be detected at concentrations as low as 0.050 µg/L. Express this concentration of atrazine in the following units: (a) Parts per billion (assume a solution density of 1.00 g/mL)
 Verified step by step guidance
Verified step by step guidance1
Understand that parts per billion (ppb) is a way to express very dilute concentrations of substances. It is defined as the mass of the solute divided by the mass of the solution, multiplied by 10^9.
Since the density of the solution is given as 1.00 g/mL, we can assume that 1 L of the solution has a mass of 1000 g (since 1 L = 1000 mL).
Convert the concentration from micrograms per liter (µg/L) to grams per liter (g/L) by recognizing that 1 µg = 10^-6 g. Therefore, 0.050 µg/L is equivalent to 0.050 x 10^-6 g/L.
Calculate the mass of atrazine in grams in 1 L of solution, which is 0.050 x 10^-6 g.
To find the concentration in ppb, divide the mass of atrazine in grams by the mass of the solution in grams (1000 g), and then multiply by 10^9 to convert to ppb.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration Units
Concentration is a measure of the amount of a substance in a given volume of solution. Common units include molarity (moles per liter), mass per volume (grams per liter), and parts per billion (ppb). Understanding how to convert between these units is essential for accurately expressing the concentration of substances in solutions.
Recommended video:
Guided course

SI Units
Parts Per Billion (ppb)
Parts per billion (ppb) is a unit of measurement used to describe very dilute concentrations of substances. It indicates how many parts of a substance are present in one billion parts of the total solution. For example, 1 ppb means 1 microgram of a substance in 1 liter of water, making it crucial for environmental and health-related assessments.
Recommended video:
Guided course

Parts per Billion (ppb)
Density of Water
The density of water is typically 1.00 g/mL at standard conditions, which simplifies calculations involving mass and volume. This property allows for straightforward conversions between mass (in grams) and volume (in milliliters or liters), facilitating the determination of concentrations in various units, including ppb.
Recommended video:
Guided course
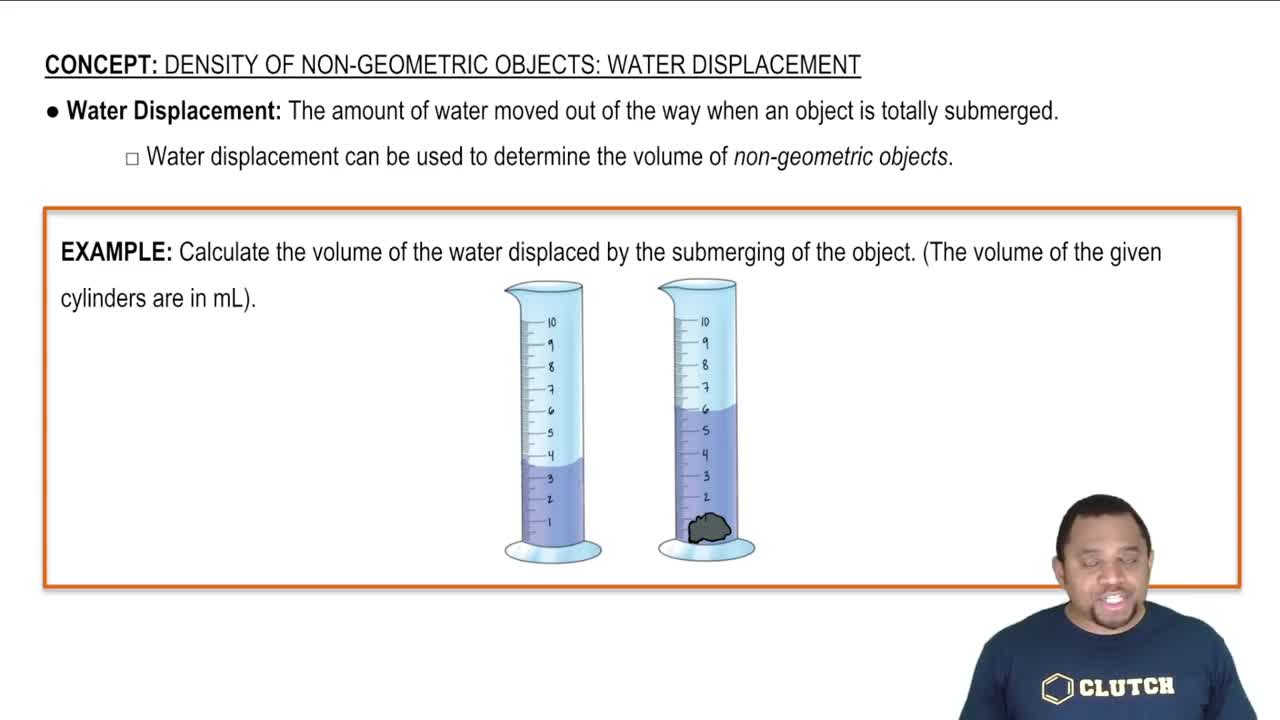
Density via Water Displacement
Related Practice
Textbook Question
2694
views
1
comments
Textbook Question
Assuming that seawater is an aqueous solution of NaCl, what is its molarity? The density of seawater is 1.025 g/mL at 20 °C, and the NaCl concentration is 3.50 mass %.
1543
views
1
rank
Textbook Question
Propranolol 1C16H21NO22, a so-called beta-blocker that is used for treatment of high blood pressure, is effective at a blood plasma concentration of 50 ng/L. Express this concen- tration of propranolol in the following units: (b) Molarity
498
views
Textbook Question
Residues of the herbicide atrazine (C8H14ClN5) in water can be detected at concentrations as low as 0.050 µg/L. Express this concentration of atrazine in the following units: (b) Molarity
351
views
Textbook Question
How would you prepare each of the following solutions?
(c) A solution of methyl alcohol (methanol) and water in which Xmethanol = 0.15 and Xwater = 0.85
391
views
Textbook Question
How would you prepare each of the following solutions? (a) 100 mL of a 155 ppm solution of urea, CH4N2O, in water
409
views
