What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
 Verified step by step guidance
Verified step by step guidance


What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
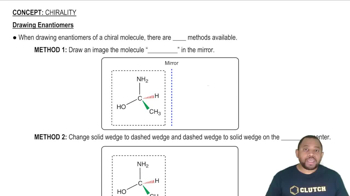
Draw all possible diastereoisomers of [Cr(C2O4)2(H2O)2]-. Which can exist as a pair of enantiomers?
Draw the three possible diastereoisomers of the triethylenetetramine complex [Co(trend)Cl2]+. Abbreviate the flexible tetradentate trien ligand H2NCH2CH2NHCH2CH2NHCH2CH2NH2 as . Which of the isomers can exist as a pair of enantiomers?
Draw the structure of all isomers of the octahedral complex [NbX2Cl4]- (X- = NCS-), and identify those that are linkage isomers.
The glycinate anion, gly-= NH2CH2CO2 -, bonds to metal ions through the N atom and one of the O atoms. Using to represent gly-, sketch the structures of the four stereoisomers of Co(gly)3.
Draw the structures of all possible diastereoisomers of an octahedral complex with the formula MA2B2C2. Which of the diastereoisomers, if any, can exist as enantiomers?