Here are the essential concepts you must grasp in order to answer the question correctly.
Crystal Field Theory
Crystal Field Theory (CFT) explains the electronic structure of transition metal complexes by considering the interaction between the metal ion's d-orbitals and the electric fields produced by surrounding ligands. In this theory, the degeneracy of d-orbitals is lifted, leading to different energy levels based on the geometry of the complex, such as octahedral or tetrahedral arrangements.
Recommended video:
The study of ligand-metal interactions helped to form Ligand Field Theory which combines CFT with MO Theory.
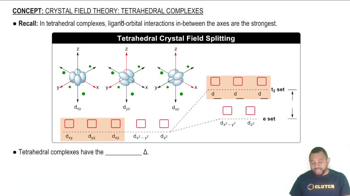
Tetrahedral Complexes
In tetrahedral complexes, the arrangement of ligands around the central metal ion creates a specific geometry where the d-orbitals split into two sets: the lower-energy e set (dxy, dyz, dzx) and the higher-energy t2 set (dx2-y2, dz2). This splitting is less pronounced than in octahedral complexes, resulting in a smaller energy difference between the orbitals, which influences electron placement and the overall stability of the complex.
Recommended video:
The crystal field splitting pattern for tetrahedral complexes has the d orbitals in between the axes as having the higher energy.
Electron Configuration and Placement
The electron configuration of a transition metal ion determines how electrons are distributed among the available d-orbitals. For [NiCl4]2+, nickel is in the +2 oxidation state, leading to a d8 configuration. In a tetrahedral field, the electrons will fill the lower-energy e orbitals first, followed by the higher-energy t2 orbitals, which is crucial for accurately drawing the crystal-field energy-level diagram.
Recommended video:
Electron Configuration Example
 Verified step by step guidance
Verified step by step guidance