Hi. In this video, we're going to talk about Gibbs free energy and equilibrium. I'm not going to lie, this is probably one of my least favorite cell biology topics, just because it's so chemistry-oriented, and it's not really my favorite thing. But don't fret because I'm leaving a lot of the heavy chemistry calculations to the chemistry courses, and we're just going to spend some time talking about how this relates to cell biology and understanding the concepts, so we know what we're talking about when we discuss topics like equilibrium.
The first concept we want to talk about is Gibbs free energy, which is a measure to determine if a chemical reaction will occur spontaneously. So, what does it mean if a reaction occurs spontaneously? Well, spontaneous reactions are those which are thermodynamically favorable. It just means that a reaction can occur without outside help. It doesn't need anything else to happen. A reaction will just occur. The reason it does that is because it's increasing the entropy, which is the disorder of the universe, and anything that really increases disorder doesn't necessarily need help because it's thermodynamically favorable.
Gibbs free energy can be calculated at a particular time point, which we really refer to as G, or it can be calculated regarding a change occurring during a reaction, which we measure as ΔG. This ΔG value changes a lot, just in a single reaction because the reaction is always moving towards equilibrium. So, these values aren't very static but instead are very dynamic depending on what point in a relation or what point in a reaction you're talking about.
Equilibrium is a state where the chemical reaction occurs equally in forward and reverse. Typically, in chemistry, we see reactants turning into products, and the reaction can occur this way or that way. Equilibrium is when this reaction is occurring equally in both directions. Why do we need to know this? How does this relate to biology? Cells do not exist at equilibrium, and instead, life depends on having reactions that are trying to reach equilibrium but not quite getting there. I think that phrase, trying to reach equilibrium but not quite getting there, expertly describes Cell Biology.
Now, let's talk about ΔG, the free energy calculation. The second calculation is the standard free energy change, which also measures the spontaneity of chemical reactions. What's the difference between the two? Well, ΔG calculates for a single direction with a known concentration of products and reactants. It measures real-life reactions and is sensitive to those changes because reactant and product concentrations are constantly changing in real-life reactions.
The standard free energy change, on the other hand, calculates spontaneity for a reaction occurring under standard conditions, which include the temperature being 25 degrees Celsius and the pressure being 1 atm. This calculation allows us to compare the thermodynamics of many reactions, so we can determine which reaction is more spontaneous under standardized conditions, rather than using ΔG which is highly dependent on changing concentrations.
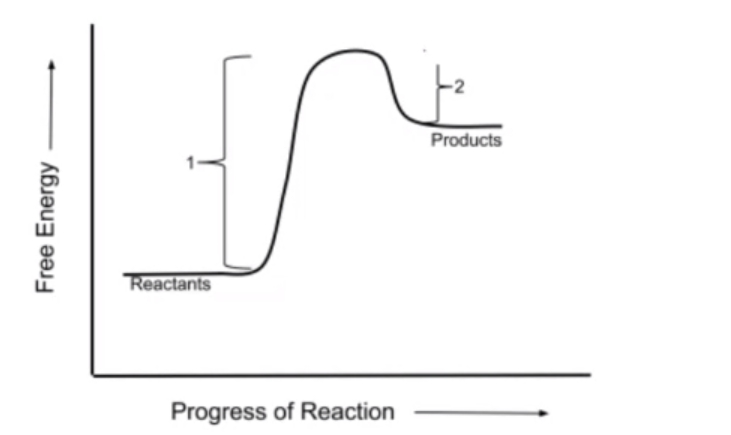
Before we turn the page, let's talk about the concepts of exergonic and endergonic reactions. You might be familiar with these terms from chemistry. Exergonic reactions release energy whereas endergonic reactions absorb energy. Because energy is being released in exergonic reactions, they are more thermodynamically favorable. In such reactions, the free energy of the products will be less than the free energy of reactants, and ΔG will be negative. For endergonic reactions, ΔG will be positive, meaning they are less thermodynamically favorable, and the free energy of the products is more than the free energy of the reactants. At equilibrium, ΔG is zero, and no reactions occur.
Now, in addition to that, let's turn the page.