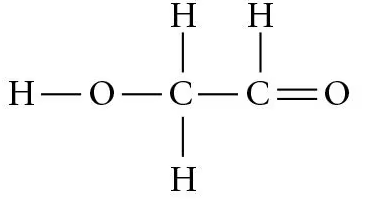
Textbook Question
We can represent atoms by listing the number of protons, neutrons, and electrons—for example, 2p+, 2p+,2n0,2e− for helium. Which of the following represents the 18O isotope of oxygen?
a. 7p+, 2n0, 9e−
b. 8p+, 10n0, 8e−
c. 9p+, 9n0, 9e−
d. 10p+, 8n0, 9e-
1685
views