In this video we're going to talk about the 3rd biogeochemical cycle in our lesson, which is the nitrogen cycle. And you can see the diagram of the nitrogen cycle down below here, which has many different colored arrows, including yellow arrows for terrestrial cycling of nitrogen, red arrows for aquatic cycling of nitrogen, and green arrows for industrial or human-related cycling of nitrogen. Now it's very important to recall that nitrogen is an essential element to all life, as nitrogen is found in the amino groups of amino acids that make up all proteins, and nitrogen is found in the nitrogenous bases of nucleotides that make up nucleic acids like DNA and RNA. And the nitrogen cycle is super important because it helps to recycle the nitrogen and ensure that nitrogen is always available in different forms to be utilized by all organisms regardless of what trophic levels they're in and what type of ecosystem they're in, terrestrial or aquatic. And so the nitrogen cycle is super important.
Without it, life would not be able to exist as we know it. And so what you should know is that the atmosphere is actually the largest reservoir of nitrogen, where 78% of the atmosphere is actually nitrogen gas or N2 gas. However, this nitrogen gas is actually biologically unusable to most organisms. What a shame. And so, this nitrogen gas can really enter ecosystems via nitrogen-fixing bacteria that can be found either in the soil or found in the root nodules of plant roots.
And so this unusable form of nitrogen is going to be converted to usable forms of nitrogen, via these nitrogen-fixing bacteria. And more usable forms of nitrogen include nitrates, or NO3-, and Ammonium or NH4+. And those are really the primary forms of nitrogen that plants and other primary producers are able to readily assimilate, absorb, and utilize. And so, let's go ahead and enlarge this image and wipe it clean so that we can take things step by step. And so, again, we know that the largest reservoir of nitrogen is the atmosphere itself, and it's going to contain 78% nitrogen gas or N2.
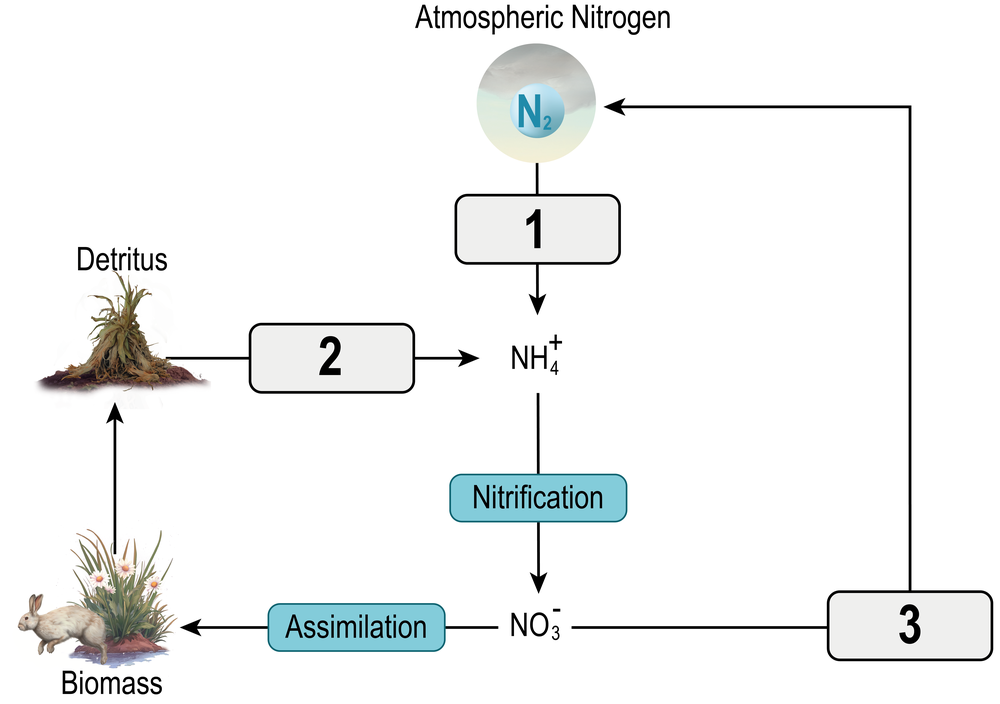
And this nitrogen gas can enter into ecosystems via nitrogen fixation, which is step number 1 of the nitrogen cycle. And nitrogen fixation can occur via bacteria in the root nodules of some plants, where that atmospheric nitrogen, N2, can be converted into more usable forms of nitrogen, such as ammonium, NH4+, which can be readily assimilated or absorbed by plants. And once plants have assimilated that nitrogen, the nitrogen can make its way up the food chain to other organisms and consumers via consumption. Now nitrogen fixation can also occur via bacteria in the soil, which can again convert the unusable nitrogen gas into more usable forms such as ammonia or NH4+.
Now in step number 2 of this process, we have a process called nitrification, which is also carried out by bacteria called nitrifying bacteria, which can convert the ammonium, NH4+, into another usable form of nitrogen called Nitrate or NO3-. So this NO3- can also be readily assimilated by plants, and once it's in the plants, it can move its way up the food chain to other organisms like this animal you see here. Now eventually, all of these animals are going to die and decompose, and the decomposition process carried out by fungi and bacteria mainly, specifically called ammonification, is the decomposition process that takes organic nitrogen in these organisms and breaks it down into raw forms of nitrogen, such as Ammonium, NH4+.
And so once this NH4+ is regenerated, it can kind of continue to go in this cycle that you see here, cycling between steps 2, 3, and 4. And the way that it can break out of the cycle is via the process of denitrification, which is step number 5 here, where nitrates can be converted back into nitrogen gas via bacteria called denitrifying bacteria. And so really that completes the terrestrial cycling of nitrogen. So now we can talk briefly about the aquatic cycling of nitrogen, and since I'm right in the way here, let me shift over so that you can see this a bit more clearly. There we go.
So over here with the aquatic cycling, really it's going to be very similar to the terrestrial cycling. In fact, we have the numbers here in the arrows that correspond with the numbers in the same processes of the terrestrial. So you can see steps 2, 3, and 4 correspond with steps 2, 3, and 4 here. And really, some of the most notable differences in the aquatic cycling is that the nitrogen fixation is carried out mainly by cyanobacteria, which are these greenish bacteria that we're showing you here in our image. And again, this steps 2, 3, and 4, which are nitrification, assimilation, and decomposition, ammonification, this cycle here can be broken, with this step number 5 of denitrification, which converts again the nitrate into nitrogen gas, completing the aquatic cycle.
Now it also turns out that humans are able to get into this cycling and get into the mix of things as well. So humans are actually able to fix nitrogen industrially to create nitrogen fertilizers, and through the burning of fossil fuels, they can also release reactive nitrogen gases that can help to complete the cycle, but these reactive nitrogen gases can also have other effects as well. For example, many of them can act as greenhouse gases that lead to global warming. Others can actually cause acidic rain, which can be devastating to some ecosystems. And some of these reactive nitrogen gases can actually dissolve the ozone layer, which is a layer in our atmosphere that protects us from solar radiation.
So humans need to be very conscious and aware of their activities and the types of reactive nitrogen gases that they're releasing. Now again, these nitrogen fertilizers can actually be added to the soil to add nitrate to the soil, so it can kind of influence the nitrogen cycle in that way. And through streams and rivers, the nitrogen fertilizers can run off into aquatic ecosystems too, contributing to aquatic cycling of nitrogen as well. Now, one of the last notes that I'll leave you off with is that nitrogen can also be fixed abiotically via lightning and volcanic activity, which is why we show you this thunderbolt right here to remind you of that abiotic fixing of nitrogen that can occur. And so, one thing that I want to draw your attention to is the numbers that you can see, again, throughout this process.
That's 1, 2, 3, 4, and 5. And the numbers that you see here in this diagram correspond really nicely with the numbers that you can see in the table on the next page. So I want to show you that here. Here it is, and you can see those numbers correspond perfectly. So you can use this table here to help facilitate your understanding and learning of the process that we just talked about.
So, here what we have are some of the reactions that occur that are associated with each of these processes. These are the 5 steps of the terrestrial nitrogen cycling and aquatic nitrogen cycling, and then here are some descriptions of each of those processes. So hopefully, this was very helpful to you. Let me move out of the way here so you can see this table, fully. And that concludes this video, so I'll see you on our next one.