In this video, we're going to begin our lesson on hydrogen bonding, and so a hydrogen bond is commonly abbreviated as just an H bond, and that's because the letter H is the chemical symbol for hydrogen. And so a hydrogen bond or an H bond is really just defined as an interaction between a highly electronegative atom such as either fluorine, oxygen, or nitrogen, and of course a hydrogen atom itself. How could you have a hydrogen bond without a hydrogen atom? And so every hydrogen bond is going to have a hydrogen atom involved. However, the highly electronegative atom will vary. Sometimes it will be either fluorine, other times it will be oxygen, and other times it will be nitrogen. But what can help you remember the highly electronegative atoms that form hydrogen bonds, is that hydrogen bonds are pretty fun and so you can think FON here sounds like fun, and so those are the 3 electronegative atoms that can help form hydrogen bonds. And so, notice down below in our image we're showing you a few examples of hydrogen bonds and we'll talk about them here shortly. But one thing to note about these hydrogen bonds is that individually the H bonds or the hydrogen bonds are really, really weak. However, collectively if you have lots and lots of hydrogen bonds forming they can be quite strong. And so these hydrogen bonds are actually important in several different areas of biology, including the properties that are found in water, which we'll get to talk more about later in our course in a different video, and hydrogen bonds are also really important for the structure of macromolecules, which we'll get to talk more about these macromolecules later in our course as well. But down below in our image, notice that we're showing you some examples of hydrogen bonds in biology. On the left, we're showing you how water molecules can form hydrogen bonds, and so notice that we have these 3 different water molecules right here H2O and notice that through hydrogen bonding they can actually form interactions between these different water molecules. And so what you'll notice is that this yellow bond right here represents the hydrogen bond and so notice that it's forming between a hydrogen atom as we indicated up above and another highly electronegative atom, in this case, it's showing oxygen here in the water molecules. And so you can see another oxygen here and another hydrogen, right here. And once again, the hydrogen bond is the bond that forms between the two. Now that is going to be very important. The hydrogen bonding in water is going to be very important for specific properties that water has that are really important for life. And once again, we'll talk about these properties of water, later in our course in a different video. But it's important to know that water does form hydrogen bonds. Now over here on the right hand side, we're showing you how nucleotides can also form hydrogen bonds. And so, over here we're showing you one nucleotide and over here we're showing you another nucleotide, and notice that between these two nucleotides there are hydrogen bonds that are forming. Notice that this first hydrogen bond up here at the top is forming between a hydrogen atom and a highly electronegative oxygen atom, but this other hydrogen bond down below right here is forming between a hydrogen atom and another highly electronegative atom of nitrogen. And so remember that the highly electronegative atom can vary. It can either be fluorine, oxygen, or nitrogen, and so here in this scenario, you can see that it's forming between Nitrogen and Hydrogen and Oxygen and Hydrogen. And so, don't worry too much about what nucleotides are right now. We'll talk more about nucleotides later in our course in a different video. For now, what you should know is that these nucleotides are going to be found in the structure of DNA. And, later in our course when we talk more about DNA, we'll revisit this idea of how hydrogen bonds form between DNA structures. But for now, this here concludes our introduction to hydrogen bonding and we'll be able to get some practice applying these concepts in our next few videos. So I'll see you all there.
- 1. Introduction to Biology2h 40m
- 2. Chemistry3h 40m
- 3. Water1h 26m
- 4. Biomolecules2h 23m
- 5. Cell Components2h 26m
- 6. The Membrane2h 31m
- 7. Energy and Metabolism2h 0m
- 8. Respiration2h 40m
- 9. Photosynthesis2h 49m
- 10. Cell Signaling59m
- 11. Cell Division2h 47m
- 12. Meiosis2h 0m
- 13. Mendelian Genetics4h 41m
- Introduction to Mendel's Experiments7m
- Genotype vs. Phenotype17m
- Punnett Squares13m
- Mendel's Experiments26m
- Mendel's Laws18m
- Monohybrid Crosses16m
- Test Crosses14m
- Dihybrid Crosses20m
- Punnett Square Probability26m
- Incomplete Dominance vs. Codominance20m
- Epistasis7m
- Non-Mendelian Genetics12m
- Pedigrees6m
- Autosomal Inheritance21m
- Sex-Linked Inheritance43m
- X-Inactivation9m
- 14. DNA Synthesis2h 27m
- 15. Gene Expression3h 20m
- 16. Regulation of Expression3h 31m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Eukaryotic Post-Translational Regulation13m
- 17. Viruses37m
- 18. Biotechnology2h 58m
- 19. Genomics17m
- 20. Development1h 5m
- 21. Evolution3h 1m
- 22. Evolution of Populations3h 52m
- 23. Speciation1h 37m
- 24. History of Life on Earth2h 6m
- 25. Phylogeny40m
- 26. Prokaryotes4h 59m
- 27. Protists1h 6m
- 28. Plants1h 22m
- 29. Fungi36m
- 30. Overview of Animals34m
- 31. Invertebrates1h 2m
- 32. Vertebrates50m
- 33. Plant Anatomy1h 3m
- 34. Vascular Plant Transport2m
- 35. Soil37m
- 36. Plant Reproduction47m
- 37. Plant Sensation and Response1h 9m
- 38. Animal Form and Function1h 19m
- 39. Digestive System10m
- 40. Circulatory System1h 57m
- 41. Immune System1h 12m
- 42. Osmoregulation and Excretion50m
- 43. Endocrine System4m
- 44. Animal Reproduction2m
- 45. Nervous System55m
- 46. Sensory Systems46m
- 47. Muscle Systems23m
- 48. Ecology3h 11m
- Introduction to Ecology20m
- Biogeography14m
- Earth's Climate Patterns50m
- Introduction to Terrestrial Biomes10m
- Terrestrial Biomes: Near Equator13m
- Terrestrial Biomes: Temperate Regions10m
- Terrestrial Biomes: Northern Regions15m
- Introduction to Aquatic Biomes27m
- Freshwater Aquatic Biomes14m
- Marine Aquatic Biomes13m
- 49. Animal Behavior28m
- 50. Population Ecology3h 41m
- Introduction to Population Ecology28m
- Population Sampling Methods23m
- Life History12m
- Population Demography17m
- Factors Limiting Population Growth14m
- Introduction to Population Growth Models22m
- Linear Population Growth6m
- Exponential Population Growth29m
- Logistic Population Growth32m
- r/K Selection10m
- The Human Population22m
- 51. Community Ecology2h 46m
- Introduction to Community Ecology2m
- Introduction to Community Interactions9m
- Community Interactions: Competition (-/-)38m
- Community Interactions: Exploitation (+/-)23m
- Community Interactions: Mutualism (+/+) & Commensalism (+/0)9m
- Community Structure35m
- Community Dynamics26m
- Geographic Impact on Communities21m
- 52. Ecosystems2h 36m
- 53. Conservation Biology24m
Hydrogen Bonding: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIA hydrogen bond, or H bond, is an interaction between a hydrogen atom and a highly electronegative atom like fluorine, oxygen, or nitrogen. These bonds are weak individually but can be strong collectively, playing a crucial role in biological processes, such as the properties of water and the structure of macromolecules like DNA. Water molecules form hydrogen bonds, which are essential for life, while nucleotides in DNA also utilize hydrogen bonds for stability. Understanding hydrogen bonding is vital for grasping key biological concepts.
Hydrogen Bonding
Video transcript
What property of the bond between a Hydrogen (H) atom and an Oxygen (O) atom in a molecule of water
makes it a polar bond?
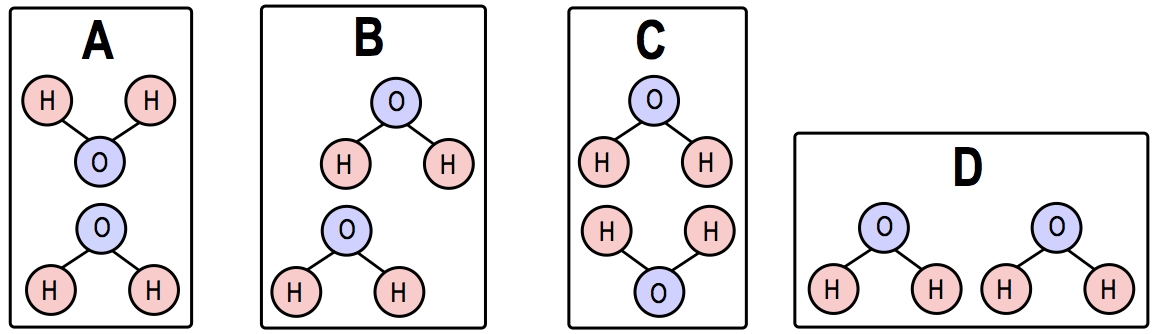
Which of the following images below is the most likely way that two water molecules would interact?
Do you want more practice?
More setsGo over this topic definitions with flashcards
More setsHere’s what students ask on this topic:
What is a hydrogen bond and how does it form?
A hydrogen bond, often abbreviated as an H bond, is an interaction between a hydrogen atom and a highly electronegative atom such as fluorine, oxygen, or nitrogen. It forms when a hydrogen atom, which is covalently bonded to one of these electronegative atoms, experiences an attraction to another electronegative atom nearby. This attraction is due to the partial positive charge on the hydrogen atom and the partial negative charge on the electronegative atom. Although individual hydrogen bonds are weak, collectively they can be quite strong and play crucial roles in biological processes, such as the properties of water and the structure of macromolecules like DNA.
 Created using AI
Created using AIWhy are hydrogen bonds important in water molecules?
Hydrogen bonds are crucial in water molecules because they contribute to water's unique properties, which are essential for life. These bonds form between the hydrogen atom of one water molecule and the oxygen atom of another. This bonding results in high cohesion, surface tension, and specific heat capacity. These properties allow water to act as a solvent, regulate temperature, and support various biological processes. For example, the high specific heat capacity helps stabilize temperatures in organisms and environments, while cohesion and surface tension enable processes like capillary action in plants.
 Created using AI
Created using AIHow do hydrogen bonds contribute to the structure of DNA?
Hydrogen bonds play a vital role in stabilizing the structure of DNA. They form between the nitrogenous bases of the DNA double helix, specifically between adenine (A) and thymine (T), and between guanine (G) and cytosine (C). Adenine and thymine form two hydrogen bonds, while guanine and cytosine form three. These bonds help hold the two strands of DNA together, ensuring the molecule's stability and integrity. This stability is crucial for DNA replication and the accurate transmission of genetic information during cell division.
 Created using AI
Created using AIWhat are some examples of highly electronegative atoms that participate in hydrogen bonding?
The highly electronegative atoms that commonly participate in hydrogen bonding are fluorine (F), oxygen (O), and nitrogen (N). These atoms have a strong tendency to attract electrons towards themselves, creating a partial negative charge. When these atoms are covalently bonded to a hydrogen atom, the hydrogen acquires a partial positive charge, allowing it to form a hydrogen bond with another electronegative atom. These interactions are fundamental in various biological molecules and processes, such as the structure of water and the stability of DNA.
 Created using AI
Created using AIHow do hydrogen bonds affect the properties of macromolecules?
Hydrogen bonds significantly influence the properties and functions of macromolecules like proteins and nucleic acids. In proteins, hydrogen bonds help stabilize secondary structures such as alpha helices and beta sheets, which are crucial for the protein's overall 3D shape and function. In nucleic acids like DNA, hydrogen bonds between complementary bases (A-T and G-C) stabilize the double helix structure, ensuring the accurate storage and transmission of genetic information. These bonds also play a role in the folding and stability of RNA molecules, affecting their function in protein synthesis and regulation.
 Created using AI
Created using AI