3. Water
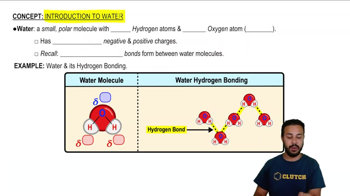
Introduction to Water
3. Water
Introduction to Water
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Individual water molecules bind to each other through:
3322views47rank - Multiple Choice
The emergent properties of water (cohesion, high heat capacity, good solvent) come from the fact that water is ______ and ______ hydrogen bond.
3278views41rank - Multiple ChoiceCells are surrounded by water, with which they are mostly filled. Which of the following occurs as a result?1295views
- Multiple ChoiceWater is a polar molecule. What does this statement mean?1567views1rank
- Textbook Question
Which of the following is not a function of water? a. dispersing nutrients throughout the body; b. helping prevent cancer; c. helping to regulate body temperature; d. helping to regulate blood pressure
1709views - Textbook QuestionWater . a. is a good solute; b. facilitates chemical reactions; c. serves as an enzyme; d. makes strong covalent bonds with other molecules; e. consists of two oxygen and one hydrogen atoms1108views
- Textbook QuestionThis chapter explains how the emergent properties of water contribute to the suitability of the environment for life. Until fairly recently, scientists assumed that other physical requirements for life included a moderate range of temperature, pH, and atmospheric pressure. That view has changed with the discovery of organisms known as extremophiles, which have been found flourishing in hot, acidic sulfur springs and around hydrothermal vents deep in the ocean. What does the existence of life in such environments say about the possibility of life on other planets?1033views