2. Chemistry
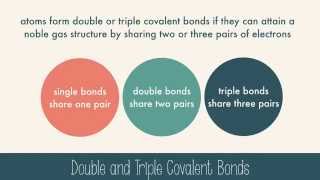
Covalent Bonds
2. Chemistry
Covalent Bonds
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
When two atoms share a pair of electrons, the bonding is referred to as:
4248views55rank - Multiple Choice
What makes a covalent bond nonpolar?
3446views39rank5comments - Multiple Choice
If a covalent bond is polar:
4030views48rank - Multiple Choice
Bonds between two atoms that are equally or similarly electronegative are ________.
3534views42rank - Textbook QuestionWhich of the following occurs when a covalent bond forms? a. Electrons in valence shells are transferred from one atom to another. b. Electrons in valence shells are shared between atoms. c. Partial charges on polar molecules interact. d. Nonpolar molecules are pushed together.by surrounding water molecules.1173views
- Textbook QuestionWhich of these molecules would you predict to have the largest number of polar covalent bonds based on their molecular formulas? a. C2H6O (ethanol) b. C2H6 (ethane) c. C2H4O2 (acetic acid) d. C3H8O (propanol)1620views
- Textbook Question
Which of these molecules would you predict to have the largest number of polar covalent bonds based on their molecular formulas? a. C2H6O (ethanol) b. C2H6 (ethane) c. C2H4O2 (acetic acid) d. C3H8O (propanol)
724views - Textbook Question
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound. a. HS b. HS2 c. H2S d. H4S
2785views