4. Biomolecules
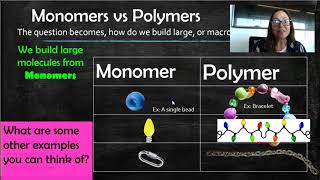
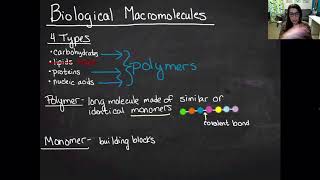
Monomers & Polymers
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following statements concerning dehydration reactions and hydrolysis is correct?
4286views46rank - Multiple Choice
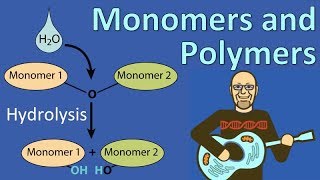
_________ bonds are formed between monomers to form a polymer.
4014views57rank - Multiple ChoiceWhat is the process by which monomers are linked together to form polymers?1537views
- Multiple ChoiceIn a hydrolysis reaction, __________, and in this process, water is __________.2025views
- Textbook Question
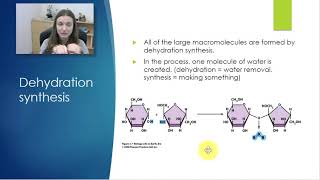
What are the defining characteristics of a condensation reaction? a. Two monomers are covalently bonded together and a water molecule is produced. b. Two monomers are covalently bonded together and a water molecule is used up. c. A polymer is broken down into monomers and a water molecule is produced. d. A polymer is broken down into monomers and a water molecule is used up.
784views - Textbook QuestionWhat are the defining characteristics of a condensation reaction? a. Two monomers are covalently bonded together and a water molecule is produced. b. Two monomers are covalently bonded together and a water molecule is used up. c. A polymer is broken down into monomers and a water molecule is produced. d. A polymer is broken down into monomers and a water molecule is used up.1855views
- Textbook Question
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions? a. C60H120O60 b. C60H102O51 c. C60H100O50 d. C60H111O51
3683views