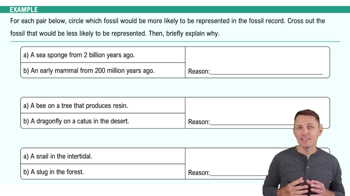
Fossils are great because they tell us all about the organisms that were alive in the past, but a lot of times we want to know when they were alive. So for that, we need to do dating of fossils. So, we're going to say here the fossil record can give us two basic types of dates. Now the first type of date we're going to call a relative date, and this type of date is pretty straightforward. It's just based on what layer in that sedimentary rock a fossil is found in.
Right? So in our image here, right, we have this sedimentary rock. We see all these different layers here. And just in simple terms, we know that this trilobite fossil has to be really old because it's found deep in the sediment. All these other layers were laid down after that trilobite was alive.
Now this dinosaur, it had to be alive more recently than the trilobite because we find it in a layer that is closer to the surface. Now this woolly mammoth, it's even higher in the sedimentary rock still, so it must have been alive relatively recently compared to those other fossils. All right, so that's great, but a lot of times we're giving pretty darn specific dates for fossils. So how do we get those? Well, we need to use what's called radiometric dates.
So radiometric dates can give us a pretty darn specific date based on the decay of radioactive isotopes. Now you have radioactivity all around you in the world. There's radioactivity in you right now. It's just at very low levels, low enough levels that it's not harmful. But because radioactivity decays, over time it goes away.
If we know how much radioactivity we started with, we can see how much is left and figure out how long it's been because radioactivity decays at a very predictable rate, and we measure that rate in something called a half-life. So the half-life is the time it takes for half the radioactivity to decay and how long that half-life is depends on the specific element. Every specific radioactive element decays at a slightly different rate. It has a slightly different half-life. Alright.
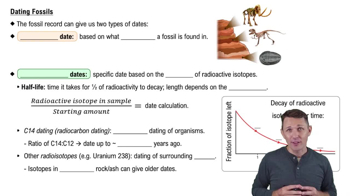
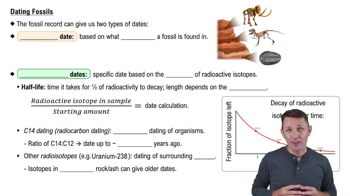
So how would this work? Because we know what the starting ratio is for all sorts of different elements, we can take the radioactivity that's left in the sample. We can compare it to what we know that starting amount of radioactivity would have had to have been, and we can use that concept of a half-life to calculate the date. Now to look at this, I'm going to look at this graph. So I'm going to move over on the screen here, and you can see we're looking at the decay of radioactive isotopes over time.
On the y-axis, we have the fraction of the isotope left going from 0 to just 100%, all of that radioactivity left. So obviously at time 0, you have all of your radioactivity there. Now after one half-life, well, you're going to lose half the radioactivity, so you have half the radioactivity left. After 2 half-lives, well, you're going to have half of what you had previously. So a half of a half, you're now down to a quarter.
After 3 half-lives, well, half of a quarter, you're now down to one-eighth of that starting amount of radioactivity. And then after 4 half-lives, you can probably see where this is going, you're now down to one-sixteenth of that original starting amount. And again, because we know the starting amount for all sorts of different elements, we can use these half-lives to calculate dates. Alright.
So now a type of radiometric dating that you may be familiar with, or you may have heard of before, is called C-14 dating or carbon-14 dating or radiocarbon dating. Now, this is great because it gives you direct dating of organisms. Right? You can pick up a bone and date that bone because living things are made of organic molecules, right? They have a lot of carbon in them.
Alright. So what we do is we take the ratio of carbon-14, that radioactive carbon. We compare it to the amount of carbon-12, and because we know the starting ratio when something was alive, well, we can use that concept of half-lives and that will give us a date up to about 60,000 years. All right, now that's great, but 60,000 years isn't that old in geologic time, right? A lot of times we're talking about 100,000, 100 of 100,000 of years.
Now the thing about carbon-14 is that it has a relatively short half-life. So after 60,000 years or so, there just isn't enough radioactivity left to give you a reliable measurement to calculate a date. So what do we do for older stuff? Well, we use other radioisotopes, right? Things like uranium-238, or you may have heard of potassium-argon dating.
Now the issue is you don't have uranium in your body. Living things don't have uranium. So what do you do? You can't date the fossil itself. You need to date the surrounding rock.
Alright. Now the issue is here though, you need to have rock that you know was new at the time it was laid down. At the time that you're finding that fossil, you need rock that was new then. Well, how do you find that? Well, what we do is we take, we measure these radioisotopes in volcanic rock or volcanic ash, and that can give us those older dates. Because when a volcano erupts, that rock gets laid down or the ash gets laid down and it ends up in our sedimentary rock. You get a layer of volcanic rock that you know had to be new at that time that it was laid down. Alright. So again, that can give us dates back to the beginning of Earth into the billions of years.
Alright. But there isn't volcanic rock, right, at every layer. So how do we do this? Well, we compare our radiometric dates and our relative dates. Right?
So you get a radiometric date. You know something was above it. You can now start to at least get estimates of when that thing was alive. And because people have done this for all the sorts of layers all over the earth with all sorts of different volcanic activity, we can get relatively specific dates for pretty much any fossil you find. Alright.
With that, we've got practices and examples after this. I'll see you there.