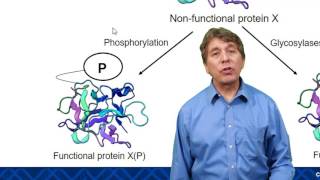
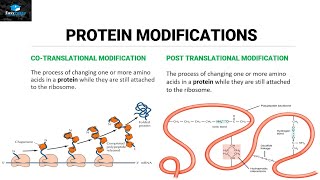
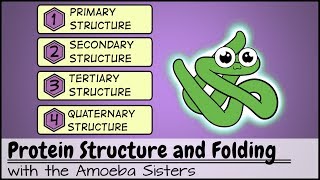
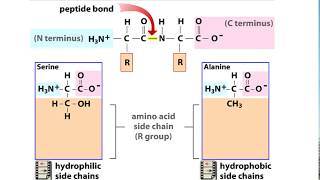
>> Life depends on proteins. These large molecules make up almost all the structures of a cell and mediate most all of its functions. Proteins are responsible for just about everything you do. For instance, enzymes are proteins that catalyze and regulate all the chemical reactions of your body. Other proteins called transport proteins move molecules into and out of your cells across the cell membrane. Some proteins act as signaling molecules that bind to protein receptors on cells and help to coordinate your body's activities. For example, the hormone insulin is a signal that causes cells to take up glucose. And the contractile proteins that make up your muscles let you dance. How can proteins perform these and so many other functions? What can you observe about the differences between these proteins? Right. They obviously differ in shape. A protein's function depends on its specific shape. Let's explore how that shape arises. A protein is made from amino acids joined by peptide bonds to form a polypeptide. The proteins of your body are composed of 20 different amino acids. You can see the different kinds indicated here by their 3-letter abbreviations. Let's take a closer look at the makeup of an individual amino acid. Each amino acid has an amino group, a carboxyl group, a hydrogen atom, and a variable group symbolized by R, and all are attached to a central carbon atom. The R groups of the various amino acids differ in their size and composition. An R group can be hydrophobic, meaning water fearing, or hydrophilic, meaning water loving. These properties help determine what shape a protein assumes. Note that leucine's R group is nonpolar and thus hydrophobic. Hydrophilic R groups may be polar, as shown here with serine, or even charged, as you can see in aspartic acid. So let's build a protein. The final functional shape of a protein is based on several superimposed levels of structure. Primary, secondary, tertiary, and in many but not all proteins, quaternary. Primary structure is the sequence of amino acids. This specific sequence is determined by instructions written in a cell's DNA. Added levels of structure, however, are required to turn a linear string of amino acids into the functional shape of a protein. There are two types of secondary structures. Portions of the chain may coil into an alpha helix. Other segments may fold back and forth, forming beta pleated sheets. These secondary structures are held together by hydrogen bonds, shown here as dotted lines between hydrogen and oxygen atoms of the polypeptide backbone. You can see secondary structures compacted in the globular three-dimensional shape of this polypeptide. The overall shape of a protein is called its tertiary structure. This shape results from interactions between R groups of the various amino acids. Hydrophobic R groups cluster in the center of a protein out of contact with water. R groups that have positive or negative charges may form ionic bonds. Hydrogen bonds can connect polar R groups, and covalent bonds may form between sulfur-containing R groups. These disulfide bridges reinforce the shape of some proteins. You just learned that various types of interactions between the amino acids that make up a polypeptide chain produce the secondary and tertiary structures that determine a protein's shape. But can changes in the chemical or physical environment disrupt a protein's shape and thus its function? For example, could an increase in temperature or a change in acidity affect a protein? Yes. A physical or chemical change in the protein's environment may disrupt the chemical interactions responsible for secondary and tertiary structure, causing the protein to unravel. This process is called denaturation. Without its specific shape, the denatured protein loses its function. Many proteins consist of a single polypeptide and thus have just primary, secondary, and tertiary structure. But some proteins have a quaternary structure in which two or more polypeptide chains are aggregated into one functional protein, such as transthyretin, as seen here. In summary, a protein's functional shape results from four levels of structure: primary, secondary, tertiary, and sometimes quaternary. [ Silence ]
Table of contents
- 1. Introduction to Biology2h 40m
- 2. Chemistry3h 40m
- 3. Water1h 26m
- 4. Biomolecules2h 23m
- 5. Cell Components2h 26m
- 6. The Membrane2h 31m
- 7. Energy and Metabolism2h 0m
- 8. Respiration2h 40m
- 9. Photosynthesis2h 49m
- 10. Cell Signaling59m
- 11. Cell Division2h 47m
- 12. Meiosis2h 0m
- 13. Mendelian Genetics4h 41m
- Introduction to Mendel's Experiments7m
- Genotype vs. Phenotype17m
- Punnett Squares13m
- Mendel's Experiments26m
- Mendel's Laws18m
- Monohybrid Crosses16m
- Test Crosses14m
- Dihybrid Crosses20m
- Punnett Square Probability26m
- Incomplete Dominance vs. Codominance20m
- Epistasis7m
- Non-Mendelian Genetics12m
- Pedigrees6m
- Autosomal Inheritance21m
- Sex-Linked Inheritance43m
- X-Inactivation9m
- 14. DNA Synthesis2h 27m
- 15. Gene Expression3h 20m
- 16. Regulation of Expression3h 31m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Eukaryotic Post-Translational Regulation13m
- 17. Viruses37m
- 18. Biotechnology2h 58m
- 19. Genomics17m
- 20. Development1h 5m
- 21. Evolution3h 1m
- 22. Evolution of Populations3h 52m
- 23. Speciation1h 37m
- 24. History of Life on Earth2h 6m
- 25. Phylogeny2h 31m
- 26. Prokaryotes4h 59m
- 27. Protists1h 12m
- 28. Plants1h 22m
- 29. Fungi36m
- 30. Overview of Animals34m
- 31. Invertebrates1h 2m
- 32. Vertebrates50m
- 33. Plant Anatomy1h 3m
- 34. Vascular Plant Transport2m
- 35. Soil37m
- 36. Plant Reproduction47m
- 37. Plant Sensation and Response1h 9m
- 38. Animal Form and Function1h 19m
- 39. Digestive System10m
- 40. Circulatory System1h 57m
- 41. Immune System1h 12m
- 42. Osmoregulation and Excretion50m
- 43. Endocrine System4m
- 44. Animal Reproduction2m
- 45. Nervous System55m
- 46. Sensory Systems46m
- 47. Muscle Systems23m
- 48. Ecology3h 11m
- Introduction to Ecology20m
- Biogeography14m
- Earth's Climate Patterns50m
- Introduction to Terrestrial Biomes10m
- Terrestrial Biomes: Near Equator13m
- Terrestrial Biomes: Temperate Regions10m
- Terrestrial Biomes: Northern Regions15m
- Introduction to Aquatic Biomes27m
- Freshwater Aquatic Biomes14m
- Marine Aquatic Biomes13m
- 49. Animal Behavior28m
- 50. Population Ecology3h 41m
- Introduction to Population Ecology28m
- Population Sampling Methods23m
- Life History12m
- Population Demography17m
- Factors Limiting Population Growth14m
- Introduction to Population Growth Models22m
- Linear Population Growth6m
- Exponential Population Growth29m
- Logistic Population Growth32m
- r/K Selection10m
- The Human Population22m
- 51. Community Ecology2h 46m
- Introduction to Community Ecology2m
- Introduction to Community Interactions9m
- Community Interactions: Competition (-/-)38m
- Community Interactions: Exploitation (+/-)23m
- Community Interactions: Mutualism (+/+) & Commensalism (+/0)9m
- Community Structure35m
- Community Dynamics26m
- Geographic Impact on Communities21m
- 52. Ecosystems2h 36m
- 53. Conservation Biology24m
4. Biomolecules
Proteins
Video duration:
5mPlay a video:
Related Videos
Related Practice