Table of contents
- 1. Introduction to Biology2h 42m
- 2. Chemistry3h 40m
- 3. Water1h 26m
- 4. Biomolecules2h 23m
- 5. Cell Components2h 26m
- 6. The Membrane2h 31m
- 7. Energy and Metabolism2h 0m
- 8. Respiration2h 40m
- 9. Photosynthesis2h 49m
- 10. Cell Signaling59m
- 11. Cell Division2h 47m
- 12. Meiosis2h 0m
- 13. Mendelian Genetics4h 44m
- Introduction to Mendel's Experiments7m
- Genotype vs. Phenotype17m
- Punnett Squares13m
- Mendel's Experiments26m
- Mendel's Laws18m
- Monohybrid Crosses19m
- Test Crosses14m
- Dihybrid Crosses20m
- Punnett Square Probability26m
- Incomplete Dominance vs. Codominance20m
- Epistasis7m
- Non-Mendelian Genetics12m
- Pedigrees6m
- Autosomal Inheritance21m
- Sex-Linked Inheritance43m
- X-Inactivation9m
- 14. DNA Synthesis2h 27m
- 15. Gene Expression3h 20m
- 16. Regulation of Expression3h 31m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Eukaryotic Post-Translational Regulation13m
- 17. Viruses37m
- 18. Biotechnology2h 58m
- 19. Genomics17m
- 20. Development1h 5m
- 21. Evolution3h 1m
- 22. Evolution of Populations3h 52m
- 23. Speciation1h 37m
- 24. History of Life on Earth2h 6m
- 25. Phylogeny2h 31m
- 26. Prokaryotes4h 59m
- 27. Protists1h 12m
- 28. Plants1h 22m
- 29. Fungi36m
- 30. Overview of Animals34m
- 31. Invertebrates1h 2m
- 32. Vertebrates50m
- 33. Plant Anatomy1h 3m
- 34. Vascular Plant Transport1h 2m
- 35. Soil37m
- 36. Plant Reproduction47m
- 37. Plant Sensation and Response1h 9m
- 38. Animal Form and Function1h 19m
- 39. Digestive System1h 10m
- 40. Circulatory System1h 57m
- 41. Immune System1h 12m
- 42. Osmoregulation and Excretion50m
- 43. Endocrine System1h 4m
- 44. Animal Reproduction1h 2m
- 45. Nervous System1h 55m
- 46. Sensory Systems46m
- 47. Muscle Systems23m
- 48. Ecology3h 11m
- Introduction to Ecology20m
- Biogeography14m
- Earth's Climate Patterns50m
- Introduction to Terrestrial Biomes10m
- Terrestrial Biomes: Near Equator13m
- Terrestrial Biomes: Temperate Regions10m
- Terrestrial Biomes: Northern Regions15m
- Introduction to Aquatic Biomes27m
- Freshwater Aquatic Biomes14m
- Marine Aquatic Biomes13m
- 49. Animal Behavior28m
- 50. Population Ecology3h 41m
- Introduction to Population Ecology28m
- Population Sampling Methods23m
- Life History12m
- Population Demography17m
- Factors Limiting Population Growth14m
- Introduction to Population Growth Models22m
- Linear Population Growth6m
- Exponential Population Growth29m
- Logistic Population Growth32m
- r/K Selection10m
- The Human Population22m
- 51. Community Ecology2h 46m
- Introduction to Community Ecology2m
- Introduction to Community Interactions9m
- Community Interactions: Competition (-/-)38m
- Community Interactions: Exploitation (+/-)23m
- Community Interactions: Mutualism (+/+) & Commensalism (+/0)9m
- Community Structure35m
- Community Dynamics26m
- Geographic Impact on Communities21m
- 52. Ecosystems2h 36m
- 53. Conservation Biology24m
4. Biomolecules
Proteins
Problem 9`
Textbook Question
Which structural level of a protein would be least affected by a disruption in hydrogen bonding? a. primary structure b. secondary structure c. tertiary structure d. quaternary structure
 Verified step by step guidance
Verified step by step guidance1
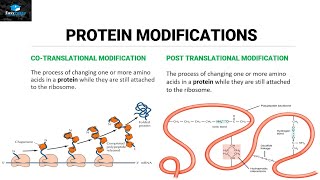
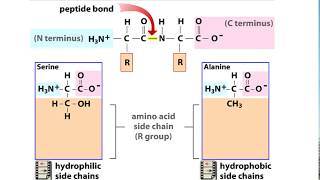
Understand the structural levels of a protein: The primary structure is the sequence of amino acids linked by peptide bonds. The secondary structure involves hydrogen bonding between the backbone of the polypeptide chain, forming alpha-helices and beta-pleated sheets. The tertiary structure is the three-dimensional folding of the protein, stabilized by various interactions, including hydrogen bonds. The quaternary structure involves the assembly of multiple polypeptide chains into a functional protein complex, also stabilized by hydrogen bonds and other interactions.
Identify the role of hydrogen bonding: Hydrogen bonds are critical for stabilizing the secondary, tertiary, and quaternary structures of a protein. However, the primary structure is determined solely by the covalent peptide bonds between amino acids and does not rely on hydrogen bonding.
Analyze the question: The problem asks which structural level would be least affected by a disruption in hydrogen bonding. Since the primary structure does not depend on hydrogen bonds, it would remain unaffected by their disruption.
Compare the other structural levels: The secondary structure (alpha-helices and beta-sheets), tertiary structure (three-dimensional folding), and quaternary structure (multi-subunit assembly) all rely on hydrogen bonding to some extent and would be affected by its disruption.
Conclude the answer: Based on the analysis, the primary structure is the structural level least affected by a disruption in hydrogen bonding.
 Verified video answer for a similar problem:
Verified video answer for a similar problem:This video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Protein Structure Levels
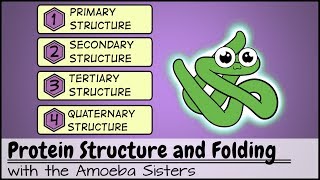
Proteins have four structural levels: primary, secondary, tertiary, and quaternary. The primary structure refers to the linear sequence of amino acids, while secondary structure involves local folding patterns like alpha helices and beta sheets. Tertiary structure is the overall 3D shape formed by interactions among various side chains, and quaternary structure is the assembly of multiple polypeptide chains into a functional protein.
Recommended video:
Guided course

Protein Structure
Hydrogen Bonding in Proteins
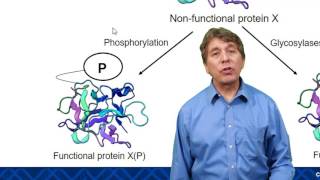
Hydrogen bonds are weak interactions that play a crucial role in stabilizing the secondary and tertiary structures of proteins. They form between polar side chains and contribute to the folding and stability of the protein's 3D shape. Disruption of hydrogen bonds can lead to denaturation, particularly affecting the secondary and tertiary structures, but not the primary structure, which is held together by covalent peptide bonds.
Recommended video:
Guided course

Hydrogen Bonding
Impact of Disruption on Protein Structure
When considering the impact of disruptions, the primary structure is least affected by hydrogen bonding disruptions because it is determined solely by the sequence of amino acids linked by peptide bonds. In contrast, secondary, tertiary, and quaternary structures rely heavily on hydrogen bonds and other non-covalent interactions for their stability. Therefore, changes in hydrogen bonding primarily influence the higher levels of protein structure.
Recommended video:
Guided course

Protein Structure
Related Videos
Related Practice