Table of contents
- 1. Introduction to Biology2h 42m
- 2. Chemistry3h 40m
- 3. Water1h 26m
- 4. Biomolecules2h 23m
- 5. Cell Components2h 26m
- 6. The Membrane2h 31m
- 7. Energy and Metabolism2h 0m
- 8. Respiration2h 40m
- 9. Photosynthesis2h 49m
- 10. Cell Signaling59m
- 11. Cell Division2h 47m
- 12. Meiosis2h 0m
- 13. Mendelian Genetics4h 44m
- Introduction to Mendel's Experiments7m
- Genotype vs. Phenotype17m
- Punnett Squares13m
- Mendel's Experiments26m
- Mendel's Laws18m
- Monohybrid Crosses19m
- Test Crosses14m
- Dihybrid Crosses20m
- Punnett Square Probability26m
- Incomplete Dominance vs. Codominance20m
- Epistasis7m
- Non-Mendelian Genetics12m
- Pedigrees6m
- Autosomal Inheritance21m
- Sex-Linked Inheritance43m
- X-Inactivation9m
- 14. DNA Synthesis2h 27m
- 15. Gene Expression3h 20m
- 16. Regulation of Expression3h 31m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Eukaryotic Post-Translational Regulation13m
- 17. Viruses37m
- 18. Biotechnology2h 58m
- 19. Genomics17m
- 20. Development1h 5m
- 21. Evolution3h 1m
- 22. Evolution of Populations3h 52m
- 23. Speciation1h 37m
- 24. History of Life on Earth2h 6m
- 25. Phylogeny2h 31m
- 26. Prokaryotes4h 59m
- 27. Protists1h 12m
- 28. Plants1h 22m
- 29. Fungi36m
- 30. Overview of Animals34m
- 31. Invertebrates1h 2m
- 32. Vertebrates50m
- 33. Plant Anatomy1h 3m
- 34. Vascular Plant Transport1h 2m
- 35. Soil37m
- 36. Plant Reproduction47m
- 37. Plant Sensation and Response1h 9m
- 38. Animal Form and Function1h 19m
- 39. Digestive System1h 10m
- 40. Circulatory System1h 57m
- 41. Immune System1h 12m
- 42. Osmoregulation and Excretion50m
- 43. Endocrine System1h 4m
- 44. Animal Reproduction1h 2m
- 45. Nervous System1h 55m
- 46. Sensory Systems46m
- 47. Muscle Systems23m
- 48. Ecology3h 11m
- Introduction to Ecology20m
- Biogeography14m
- Earth's Climate Patterns50m
- Introduction to Terrestrial Biomes10m
- Terrestrial Biomes: Near Equator13m
- Terrestrial Biomes: Temperate Regions10m
- Terrestrial Biomes: Northern Regions15m
- Introduction to Aquatic Biomes27m
- Freshwater Aquatic Biomes14m
- Marine Aquatic Biomes13m
- 49. Animal Behavior28m
- 50. Population Ecology3h 41m
- Introduction to Population Ecology28m
- Population Sampling Methods23m
- Life History12m
- Population Demography17m
- Factors Limiting Population Growth14m
- Introduction to Population Growth Models22m
- Linear Population Growth6m
- Exponential Population Growth29m
- Logistic Population Growth32m
- r/K Selection10m
- The Human Population22m
- 51. Community Ecology2h 46m
- Introduction to Community Ecology2m
- Introduction to Community Interactions9m
- Community Interactions: Competition (-/-)38m
- Community Interactions: Exploitation (+/-)23m
- Community Interactions: Mutualism (+/+) & Commensalism (+/0)9m
- Community Structure35m
- Community Dynamics26m
- Geographic Impact on Communities21m
- 52. Ecosystems2h 36m
- 53. Conservation Biology24m
3. Water
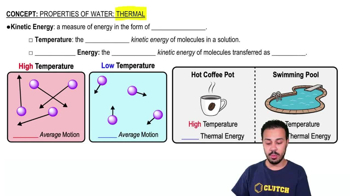
Properties of Water- Thermal
Problem 5`
Textbook Question
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)
a. 50°C
b. 5°C
c. 100°C
d. 10°C
 Verified step by step guidance
Verified step by step guidance1
First, understand the relationship between calories and energy. 1 kcal (kilocalorie) is equivalent to 1000 calories, and it is also equivalent to 4184 joules. Therefore, 500 kcal is equal to 500 * 4184 joules.
Next, recognize that the specific heat capacity of water is approximately 4.184 J/g°C. This means it takes 4.184 joules to raise the temperature of 1 gram of water by 1°C.
Calculate the total mass of the water. Since 1 liter of water weighs about 1 kg, a 50-L container of water would weigh approximately 50 kg, which is equivalent to 50,000 grams.
Use the formula for heat transfer: \( Q = m \, c \, \Delta T \), where \( Q \) is the heat energy in joules, \( m \) is the mass in grams, \( c \) is the specific heat capacity, and \( \Delta T \) is the change in temperature in °C. Rearrange the formula to solve for \( \Delta T \): \( \Delta T = \frac{Q}{m \, c} \).
Substitute the values into the formula: \( \Delta T = \frac{500 \, \text{kcal} \, \times \, 4184 \, \text{J/kcal}}{50,000 \, \text{g} \, \times \, 4.184 \, \text{J/g°C}} \). Calculate \( \Delta T \) to find the approximate increase in temperature of the water.
 Verified video answer for a similar problem:
Verified video answer for a similar problem:This video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Calorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. It involves using a calorimeter to determine the heat transfer between substances. In this context, it helps calculate how much heat from burning the pizza can increase the temperature of water, using the formula Q = mcΔT, where Q is heat energy, m is mass, c is specific heat capacity, and ΔT is the change in temperature.
Specific Heat Capacity
Specific heat capacity is the amount of heat required to change the temperature of a unit mass of a substance by one degree Celsius. For water, this value is approximately 4.18 J/g°C. Understanding this concept is crucial for calculating the temperature change in the water when a known amount of heat is applied, as it determines how much energy is needed to raise the temperature of the water.
Recommended video:
Guided course

Water’s High Specific Heat
Energy Conversion
Energy conversion involves transforming energy from one form to another, such as chemical energy in food to thermal energy. In this scenario, the pizza's caloric content (500 kcal) is converted into heat energy. Knowing that 1 kcal equals 4184 joules allows us to calculate the total energy available to heat the water, which is essential for determining the temperature increase.
Recommended video:
Guided course

Introduction to Energy

 4:07m
4:07mWatch next
Master Properties of Water- Thermal with a bite sized video explanation from Jason
Start learningRelated Videos
Related Practice