Table of contents
- 1. Introduction to Biology2h 40m
- 2. Chemistry3h 40m
- 3. Water1h 26m
- 4. Biomolecules2h 23m
- 5. Cell Components2h 26m
- 6. The Membrane2h 31m
- 7. Energy and Metabolism2h 0m
- 8. Respiration2h 40m
- 9. Photosynthesis2h 49m
- 10. Cell Signaling59m
- 11. Cell Division2h 47m
- 12. Meiosis2h 0m
- 13. Mendelian Genetics4h 41m
- Introduction to Mendel's Experiments7m
- Genotype vs. Phenotype17m
- Punnett Squares13m
- Mendel's Experiments26m
- Mendel's Laws18m
- Monohybrid Crosses16m
- Test Crosses14m
- Dihybrid Crosses20m
- Punnett Square Probability26m
- Incomplete Dominance vs. Codominance20m
- Epistasis7m
- Non-Mendelian Genetics12m
- Pedigrees6m
- Autosomal Inheritance21m
- Sex-Linked Inheritance43m
- X-Inactivation9m
- 14. DNA Synthesis2h 27m
- 15. Gene Expression3h 20m
- 16. Regulation of Expression3h 31m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Eukaryotic Post-Translational Regulation13m
- 17. Viruses37m
- 18. Biotechnology2h 58m
- 19. Genomics17m
- 20. Development1h 5m
- 21. Evolution3h 1m
- 22. Evolution of Populations3h 52m
- 23. Speciation1h 37m
- 24. History of Life on Earth2h 6m
- 25. Phylogeny2h 31m
- 26. Prokaryotes4h 59m
- 27. Protists1h 12m
- 28. Plants1h 22m
- 29. Fungi36m
- 30. Overview of Animals34m
- 31. Invertebrates1h 2m
- 32. Vertebrates50m
- 33. Plant Anatomy1h 3m
- 34. Vascular Plant Transport2m
- 35. Soil37m
- 36. Plant Reproduction47m
- 37. Plant Sensation and Response1h 9m
- 38. Animal Form and Function1h 19m
- 39. Digestive System10m
- 40. Circulatory System1h 57m
- 41. Immune System1h 12m
- 42. Osmoregulation and Excretion50m
- 43. Endocrine System4m
- 44. Animal Reproduction2m
- 45. Nervous System55m
- 46. Sensory Systems46m
- 47. Muscle Systems23m
- 48. Ecology3h 11m
- Introduction to Ecology20m
- Biogeography14m
- Earth's Climate Patterns50m
- Introduction to Terrestrial Biomes10m
- Terrestrial Biomes: Near Equator13m
- Terrestrial Biomes: Temperate Regions10m
- Terrestrial Biomes: Northern Regions15m
- Introduction to Aquatic Biomes27m
- Freshwater Aquatic Biomes14m
- Marine Aquatic Biomes13m
- 49. Animal Behavior28m
- 50. Population Ecology3h 41m
- Introduction to Population Ecology28m
- Population Sampling Methods23m
- Life History12m
- Population Demography17m
- Factors Limiting Population Growth14m
- Introduction to Population Growth Models22m
- Linear Population Growth6m
- Exponential Population Growth29m
- Logistic Population Growth32m
- r/K Selection10m
- The Human Population22m
- 51. Community Ecology2h 46m
- Introduction to Community Ecology2m
- Introduction to Community Interactions9m
- Community Interactions: Competition (-/-)38m
- Community Interactions: Exploitation (+/-)23m
- Community Interactions: Mutualism (+/+) & Commensalism (+/0)9m
- Community Structure35m
- Community Dynamics26m
- Geographic Impact on Communities21m
- 52. Ecosystems2h 36m
- 53. Conservation Biology24m
6. The Membrane
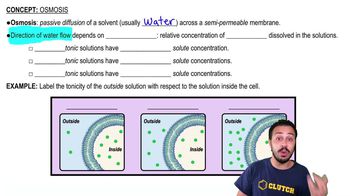
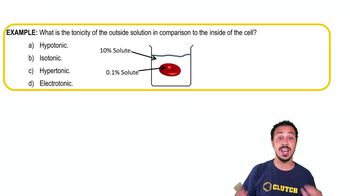
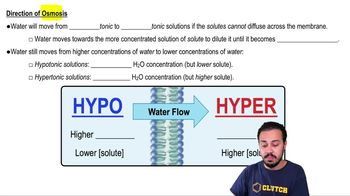
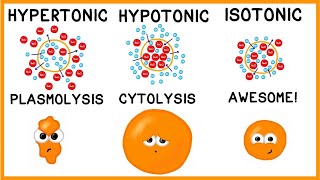
Osmosis
Problem 6
Textbook Question
Textbook QuestionDRAW IT An artificial 'cell' consisting of an aqueous solution enclosed in a selectively permeable membrane is immersed in a beaker containing a different solution, the 'environment,' as shown in the accompanying diagram. The membrane is permeable to water and to the simple sugars glucose and fructose but impermeable to the disaccharide sucrose. a. Draw solid arrows to indicate the net movement of solutes into and/or out of the cell. b. Is the solution outside the cell isotonic, hypotonic, or hypertonic? c. Draw a dashed arrow to show the net osmosis, if any. d. Will the artificial cell become more flaccid, more turgid, or stay the same? e. Eventually, will the two solutions have the same or different solute concentrations?
 Verified step by step guidance
Verified step by step guidance1
1. To draw the net movement of solutes, you need to understand the concept of diffusion. Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration. Since the membrane is permeable to glucose, fructose, and water, these molecules will move from where they are in higher concentration to where they are in lower concentration. If the concentration of these molecules is higher inside the artificial cell, draw arrows from the cell to the environment. If the concentration is higher in the environment, draw arrows from the environment to the cell.
2. To determine if the solution outside the cell is isotonic, hypotonic, or hypertonic, you need to compare the total solute concentration inside the cell with that in the environment. If the concentration is the same, the solution is isotonic. If the concentration is lower outside the cell, the solution is hypotonic. If the concentration is higher outside the cell, the solution is hypertonic.
3. Osmosis is the movement of water across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. To show the net osmosis, draw a dashed arrow from the area of lower solute concentration to the area of higher solute concentration. If the solute concentration is the same on both sides, there will be no net osmosis.
4. Whether the artificial cell becomes more flaccid, more turgid, or stays the same depends on the net movement of water. If water moves into the cell (from a hypotonic environment), the cell will become more turgid. If water moves out of the cell (into a hypertonic environment), the cell will become more flaccid. If there is no net movement of water (in an isotonic environment), the cell will stay the same.
5. Eventually, the two solutions will have the same solute concentrations. This is because the membrane is permeable to water and the simple sugars glucose and fructose. These molecules will continue to move across the membrane until the concentrations on both sides are equal, a state known as equilibrium.
Recommended similar problem, with video answer:
Verified Solution
Video duration:
4mPlay a video:
Related Videos
Related Practice