Titrations are a fundamental laboratory technique used to measure pH changes in acid-based solutions, primarily focusing on weak acids, which are prevalent in biological systems. The process involves a titrant, a solution of known concentration, being gradually added to an analyte solution, which has an unknown concentration. The goal is to reach the point of neutralization, where the moles of titrant equal the moles of analyte, indicated by a visible color change.
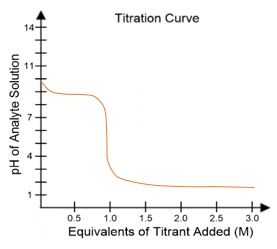
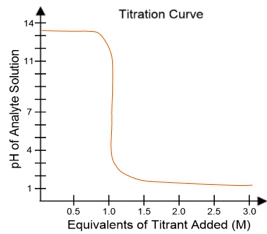
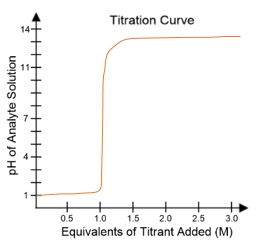
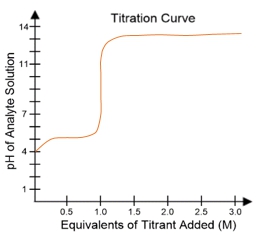
In a typical titration setup, the analyte solution is contained in an Erlenmeyer flask, while the titrant is held in a burette. A pH electrode continuously measures the pH of the analyte as titrant is added. The titration curve, which plots the pH of the analyte against the amount of titrant added, is crucial for visualizing the process. The equivalence point, or endpoint, occurs when the moles of titrant added are equal to the moles of analyte, typically resulting in a pH of 7 for strong acid-strong base titrations.
For example, when titrating a strong acid like hydrochloric acid (HCl) with a strong base such as sodium hydroxide (NaOH), the titration curve will show a low initial pH, indicating the presence of a strong acid. As titrant is added, the pH will rise sharply at the equivalence point, confirming the neutralization of the acid. The equivalence point is marked at 1 molar equivalent of titrant, meaning 100% of the analyte has been neutralized.
Understanding titration curves is essential for predicting the type of titration being performed. The characteristics of the curve, including the initial pH and the pH at the equivalence point, provide insights into the nature of the acids and bases involved. This knowledge is particularly valuable in biochemistry, where weak acids play a significant role in various biological processes.