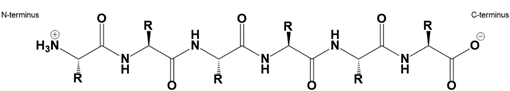
Amino acids, the building blocks of proteins, can link together through a specific type of bond known as a peptide bond. This bond is an amide covalent linkage formed between the carboxyl group of one amino acid and the amino group of another. It is important to note that the total number of peptide bonds in a polypeptide chain is always one less than the total number of amino acids present. For instance, if there are two amino acids, only one peptide bond is formed.
The formation of peptide bonds occurs through a process called dehydration synthesis, which is an endergonic reaction requiring energy input, typically in the form of ATP. During this reaction, a water molecule is released as the amino acids combine, hence the term "dehydration." Conversely, the breakdown of peptide bonds occurs through hydrolysis, an exergonic process that involves the addition of water to cleave the bond, resulting in the separation of the amino acids.
In a dehydration synthesis reaction, two free amino acids, such as glycine and alanine, undergo a reaction where the carboxyl group of glycine interacts with the amino group of alanine. This interaction leads to the formation of a peptide bond, represented by a carbonyl group linked to a nitrogen atom, and the release of a water molecule. The alpha carbon, which is the central carbon atom of each amino acid, plays a crucial role in this process.
On the other hand, hydrolysis involves adding water to the peptide bond, which initiates the cleavage of the bond, resulting in the formation of two separate amino acids. Although hydrolysis is an exergonic process, it occurs slowly due to a significant energy barrier that must be overcome for the reaction to proceed. This stability of peptide bonds is essential for the integrity of proteins, allowing them to maintain their structure despite the spontaneous nature of hydrolysis.
In summary, understanding the dynamics of peptide bond formation and breakdown is crucial for grasping how proteins are synthesized and maintained within biological systems. The interplay between dehydration synthesis and hydrolysis highlights the energy requirements and stability of these essential biomolecules.