In this video, we're going to begin our discussion on the lock and key and the induced fit models. So it turns out that there are 2 main models for enzyme substrate specificity, or 2 main models for how the enzyme interacts with the substrate to form the enzyme substrate complex. And so the first main model is the lock and key model, whereas the second main model is the induced fit model. And so in this video, we're only going to talk about the lock and key model, but in our next lesson video, we'll talk about the induced fit model. And so with the lock and key model, the shape of the active site on the enzyme is rigid and perfectly complementary to the substrate's shape. Complementary to the substrate's shape. And so what this means is that in the lock and key model, the shape of the active site is perfectly suited to the shape of the substrate to create a perfect puzzle piece-like fit. And so if we take a look at our example down below of the lock and key model, notice that our enzyme is in purple here and our substrate is this key shape here. And so, in the lock and key model, the substrate acts as the key and the enzyme acts as the lock. And in the lock and key model, the active site of the enzyme takes on a shape that is perfectly complementary to the shape of the substrate. So when the enzyme substrate complex forms, over here, you can see that the substrate fits into the active site like a perfect puzzle piece-like fit. And so there's no need for the enzyme or the substrate to have to change conformations in order for the enzyme substrate complex to form. And so ultimately, what this means is that in the lock and key model, the enzyme substrate complex that forms is going to be very stable, almost way too stable in some cases. And so over the years, the lock and key model has actually become a less likely model for enzyme catalysis, and this has to do with the fact that the enzyme substrate complex becomes so stabilized with this lock and key model. And, ultimately, the stabilization of the enzyme substrate complex leads to the energy of activation either staying exactly the same, or even potentially increasing in comparison to the uncatalyzed reaction. And recall, that's the exact opposite effect that we want enzymes to have on reactions. We want enzymes to decrease the energy of activation, not to keep the energy of activation the same or even increase them, and that is again why the lock and key model is a less likely model. So, let's take a look down below at our energy diagram that we have of the lock and key model, where we have the free energy on the y-axis and the reaction coordinate on the x-axis. And notice that the dotted line curve right here represents the uncatalyzed reaction, whereas the red curve here represents the enzyme catalyzed reaction. And notice that this black line right here, this black arrow, represents the energy of activation for the uncatalyzed reaction and typically, enzymes are supposed to decrease the energy of activation for a reaction, but notice that the enzyme catalyzed activation energy represented by this red arrow here is pretty much exactly the same size as the uncatalyzed energy of activation. And that has to do with the fact that the lock and key model stabilizes the energy, the enzyme substrate complex, the energy of the enzyme substrate complex. So this low energy of the enzyme substrate complex ultimately leads to the energy of activation, this energy barrier represented by this red arrow here, being exactly the same size or becoming even larger depending on how well this transition state is stabilized. And so, ultimately, this is why the lock and key model is a less likely model for enzyme catalysis. And so a more likely model for enzyme catalysis is the induced fit model, which we'll talk about in our next lesson video. So I'll see you guys there.
- 1. Introduction to Biochemistry4h 34m
- What is Biochemistry?5m
- Characteristics of Life12m
- Abiogenesis13m
- Nucleic Acids16m
- Proteins12m
- Carbohydrates8m
- Lipids10m
- Taxonomy10m
- Cell Organelles12m
- Endosymbiotic Theory11m
- Central Dogma22m
- Functional Groups15m
- Chemical Bonds13m
- Organic Chemistry31m
- Entropy17m
- Second Law of Thermodynamics11m
- Equilibrium Constant10m
- Gibbs Free Energy37m
- 2. Water3h 23m
- 3. Amino Acids8h 10m
- Amino Acid Groups8m
- Amino Acid Three Letter Code13m
- Amino Acid One Letter Code37m
- Amino Acid Configuration20m
- Essential Amino Acids14m
- Nonpolar Amino Acids21m
- Aromatic Amino Acids14m
- Polar Amino Acids16m
- Charged Amino Acids40m
- How to Memorize Amino Acids1h 7m
- Zwitterion33m
- Non-Ionizable Vs. Ionizable R-Groups11m
- Isoelectric Point10m
- Isoelectric Point of Amino Acids with Ionizable R-Groups51m
- Titrations of Amino Acids with Non-Ionizable R-Groups44m
- Titrations of Amino Acids with Ionizable R-Groups38m
- Amino Acids and Henderson-Hasselbalch44m
- 4. Protein Structure10h 4m
- Peptide Bond18m
- Primary Structure of Protein31m
- Altering Primary Protein Structure15m
- Drawing a Peptide44m
- Determining Net Charge of a Peptide42m
- Isoelectric Point of a Peptide37m
- Approximating Protein Mass7m
- Peptide Group22m
- Ramachandran Plot26m
- Atypical Ramachandran Plots12m
- Alpha Helix15m
- Alpha Helix Pitch and Rise20m
- Alpha Helix Hydrogen Bonding24m
- Alpha Helix Disruption23m
- Beta Strand12m
- Beta Sheet12m
- Antiparallel and Parallel Beta Sheets39m
- Beta Turns26m
- Tertiary Structure of Protein16m
- Protein Motifs and Domains23m
- Denaturation14m
- Anfinsen Experiment20m
- Protein Folding34m
- Chaperone Proteins19m
- Prions4m
- Quaternary Structure15m
- Simple Vs. Conjugated Proteins10m
- Fibrous and Globular Proteins11m
- 5. Protein Techniques14h 5m
- Protein Purification7m
- Protein Extraction5m
- Differential Centrifugation15m
- Salting Out18m
- Dialysis9m
- Column Chromatography11m
- Ion-Exchange Chromatography35m
- Anion-Exchange Chromatography38m
- Size Exclusion Chromatography28m
- Affinity Chromatography16m
- Specific Activity16m
- HPLC29m
- Spectrophotometry51m
- Native Gel Electrophoresis23m
- SDS-PAGE34m
- SDS-PAGE Strategies16m
- Isoelectric Focusing17m
- 2D-Electrophoresis23m
- Diagonal Electrophoresis29m
- Mass Spectrometry12m
- Mass Spectrum47m
- Tandem Mass Spectrometry16m
- Peptide Mass Fingerprinting16m
- Overview of Direct Protein Sequencing30m
- Amino Acid Hydrolysis10m
- FDNB26m
- Chemical Cleavage of Bonds29m
- Peptidases1h 6m
- Edman Degradation30m
- Edman Degradation Sequenator and Sequencing Data Analysis4m
- Edman Degradation Reaction Efficiency20m
- Ordering Cleaved Fragments21m
- Strategy for Ordering Cleaved Fragments58m
- Indirect Protein Sequencing Via Geneomic Analyses24m
- 6. Enzymes and Enzyme Kinetics13h 38m
- Enzymes24m
- Enzyme-Substrate Complex17m
- Lock and Key Vs. Induced Fit Models23m
- Optimal Enzyme Conditions9m
- Activation Energy24m
- Types of Enzymes41m
- Cofactor15m
- Catalysis19m
- Electrostatic and Metal Ion Catalysis11m
- Covalent Catalysis18m
- Reaction Rate10m
- Enzyme Kinetics24m
- Rate Constants and Rate Law35m
- Reaction Orders52m
- Rate Constant Units11m
- Initial Velocity31m
- Vmax Enzyme27m
- Km Enzyme42m
- Steady-State Conditions25m
- Michaelis-Menten Assumptions18m
- Michaelis-Menten Equation52m
- Lineweaver-Burk Plot43m
- Michaelis-Menten vs. Lineweaver-Burk Plots20m
- Shifting Lineweaver-Burk Plots37m
- Calculating Vmax40m
- Calculating Km31m
- Kcat46m
- Specificity Constant1h 1m
- 7. Enzyme Inhibition and Regulation 8h 42m
- Enzyme Inhibition13m
- Irreversible Inhibition12m
- Reversible Inhibition9m
- Inhibition Constant26m
- Degree of Inhibition15m
- Apparent Km and Vmax29m
- Inhibition Effects on Reaction Rate10m
- Competitive Inhibition52m
- Uncompetitive Inhibition33m
- Mixed Inhibition40m
- Noncompetitive Inhibition26m
- Recap of Reversible Inhibition37m
- Allosteric Regulation7m
- Allosteric Kinetics17m
- Allosteric Enzyme Conformations33m
- Allosteric Effectors18m
- Concerted (MWC) Model25m
- Sequential (KNF) Model20m
- Negative Feedback13m
- Positive Feedback15m
- Post Translational Modification14m
- Ubiquitination19m
- Phosphorylation16m
- Zymogens13m
- 8. Protein Function 9h 41m
- Introduction to Protein-Ligand Interactions15m
- Protein-Ligand Equilibrium Constants22m
- Protein-Ligand Fractional Saturation32m
- Myoglobin vs. Hemoglobin27m
- Heme Prosthetic Group31m
- Hemoglobin Cooperativity23m
- Hill Equation21m
- Hill Plot42m
- Hemoglobin Binding in Tissues & Lungs31m
- Hemoglobin Carbonation & Protonation19m
- Bohr Effect23m
- BPG Regulation of Hemoglobin24m
- Fetal Hemoglobin6m
- Sickle Cell Anemia24m
- Chymotrypsin18m
- Chymotrypsin's Catalytic Mechanism38m
- Glycogen Phosphorylase21m
- Liver vs Muscle Glycogen Phosphorylase21m
- Antibody35m
- ELISA15m
- Motor Proteins14m
- Skeletal Muscle Anatomy22m
- Skeletal Muscle Contraction45m
- 9. Carbohydrates7h 49m
- Carbohydrates19m
- Monosaccharides15m
- Stereochemistry of Monosaccharides33m
- Monosaccharide Configurations32m
- Cyclic Monosaccharides20m
- Hemiacetal vs. Hemiketal19m
- Anomer14m
- Mutarotation13m
- Pyranose Conformations23m
- Common Monosaccharides33m
- Derivatives of Monosaccharides21m
- Reducing Sugars21m
- Reducing Sugars Tests19m
- Glycosidic Bond48m
- Disaccharides40m
- Glycoconjugates12m
- Polysaccharide7m
- Cellulose7m
- Chitin8m
- Peptidoglycan12m
- Starch13m
- Glycogen14m
- Lectins16m
- 10. Lipids5h 49m
- Lipids15m
- Fatty Acids30m
- Fatty Acid Nomenclature11m
- Omega-3 Fatty Acids12m
- Triacylglycerols11m
- Glycerophospholipids24m
- Sphingolipids13m
- Sphingophospholipids8m
- Sphingoglycolipids12m
- Sphingolipid Recap22m
- Waxes5m
- Eicosanoids19m
- Isoprenoids9m
- Steroids14m
- Steroid Hormones11m
- Lipid Vitamins19m
- Comprehensive Final Lipid Map13m
- Biological Membranes16m
- Physical Properties of Biological Membranes18m
- Types of Membrane Proteins8m
- Integral Membrane Proteins16m
- Peripheral Membrane Proteins12m
- Lipid-Linked Membrane Proteins21m
- 11. Biological Membranes and Transport 6h 37m
- Biological Membrane Transport21m
- Passive vs. Active Transport18m
- Passive Membrane Transport21m
- Facilitated Diffusion8m
- Erythrocyte Facilitated Transporter Models30m
- Membrane Transport of Ions29m
- Primary Active Membrane Transport15m
- Sodium-Potassium Ion Pump20m
- SERCA: Calcium Ion Pump10m
- ABC Transporters12m
- Secondary Active Membrane Transport12m
- Glucose Active Symporter Model19m
- Endocytosis & Exocytosis18m
- Neurotransmitter Release23m
- Summary of Membrane Transport21m
- Thermodynamics of Membrane Diffusion: Uncharged Molecule51m
- Thermodynamics of Membrane Diffusion: Charged Ion1h 1m
- 12. Biosignaling9h 45m
- Introduction to Biosignaling44m
- G protein-Coupled Receptors32m
- Stimulatory Adenylate Cyclase GPCR Signaling42m
- cAMP & PKA28m
- Inhibitory Adenylate Cyclase GPCR Signaling29m
- Drugs & Toxins Affecting GPCR Signaling20m
- Recap of Adenylate Cyclase GPCR Signaling5m
- Phosphoinositide GPCR Signaling58m
- PSP Secondary Messengers & PKC27m
- Recap of Phosphoinositide Signaling7m
- Receptor Tyrosine Kinases26m
- Insulin28m
- Insulin Receptor23m
- Insulin Signaling on Glucose Metabolism57m
- Recap Of Insulin Signaling in Glucose Metabolism6m
- Insulin Signaling as a Growth Factor1h 1m
- Recap of Insulin Signaling As A Growth Factor9m
- Recap of Insulin Signaling1m
- Jak-Stat Signaling25m
- Lipid Hormone Signaling15m
- Summary of Biosignaling13m
- Signaling Defects & Cancer20m
- Review 1: Nucleic Acids, Lipids, & Membranes2h 47m
- Nucleic Acids 19m
- Nucleic Acids 211m
- Nucleic Acids 34m
- Nucleic Acids 44m
- DNA Sequencing 19m
- DNA Sequencing 211m
- Lipids 111m
- Lipids 24m
- Membrane Structure 110m
- Membrane Structure 29m
- Membrane Transport 18m
- Membrane Transport 24m
- Membrane Transport 36m
- Practice - Nucleic Acids 111m
- Practice - Nucleic Acids 23m
- Practice - Nucleic Acids 39m
- Lipids11m
- Practice - Membrane Structure 17m
- Practice - Membrane Structure 25m
- Practice - Membrane Transport 16m
- Practice - Membrane Transport 26m
- Review 2: Biosignaling, Glycolysis, Gluconeogenesis, & PP-Pathway3h 12m
- Biosignaling 19m
- Biosignaling 219m
- Biosignaling 311m
- Biosignaling 49m
- Glycolysis 17m
- Glycolysis 27m
- Glycolysis 38m
- Glycolysis 410m
- Fermentation6m
- Gluconeogenesis 18m
- Gluconeogenesis 27m
- Pentose Phosphate Pathway15m
- Practice - Biosignaling13m
- Practice - Bioenergetics 110m
- Practice - Bioenergetics 216m
- Practice - Glycolysis 111m
- Practice - Glycolysis 27m
- Practice - Gluconeogenesis5m
- Practice - Pentose Phosphate Path6m
- Review 3: Pyruvate & Fatty Acid Oxidation, Citric Acid Cycle, & Glycogen Metabolism2h 26m
- Pyruvate Oxidation9m
- Citric Acid Cycle 114m
- Citric Acid Cycle 27m
- Citric Acid Cycle 37m
- Citric Acid Cycle 411m
- Metabolic Regulation 18m
- Metabolic Regulation 213m
- Glycogen Metabolism 16m
- Glycogen Metabolism 28m
- Fatty Acid Oxidation 111m
- Fatty Acid Oxidation 28m
- Citric Acid Cycle Practice 17m
- Citric Acid Cycle Practice 26m
- Citric Acid Cycle Practice 32m
- Glucose and Glycogen Regulation Practice 14m
- Glucose and Glycogen Regulation Practice 26m
- Fatty Acid Oxidation Practice 14m
- Fatty Acid Oxidation Practice 27m
- Review 4: Amino Acid Oxidation, Oxidative Phosphorylation, & Photophosphorylation1h 48m
- Amino Acid Oxidation 15m
- Amino Acid Oxidation 211m
- Oxidative Phosphorylation 18m
- Oxidative Phosphorylation 210m
- Oxidative Phosphorylation 310m
- Oxidative Phosphorylation 47m
- Photophosphorylation 15m
- Photophosphorylation 29m
- Photophosphorylation 310m
- Practice: Amino Acid Oxidation 12m
- Practice: Amino Acid Oxidation 22m
- Practice: Oxidative Phosphorylation 15m
- Practice: Oxidative Phosphorylation 24m
- Practice: Oxidative Phosphorylation 35m
- Practice: Photophosphorylation 15m
- Practice: Photophosphorylation 21m
Lock and Key Vs. Induced Fit Models: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIThe lock and key model describes enzyme-substrate interaction as a rigid fit, where the enzyme's active site is perfectly complementary to the substrate's shape, leading to a stable enzyme-substrate complex. However, this model is less likely because it does not effectively lower the activation energy. In contrast, the induced fit model allows for conformational changes in both the enzyme and substrate, prioritizing stabilization of the transition state, which decreases the activation energy and enhances reaction rates, making it a more accurate representation of enzyme catalysis.
Lock-and-Key Vs. Induced Fit Models
Video transcript
Lock and Key Vs. Induced Fit Models
Video transcript
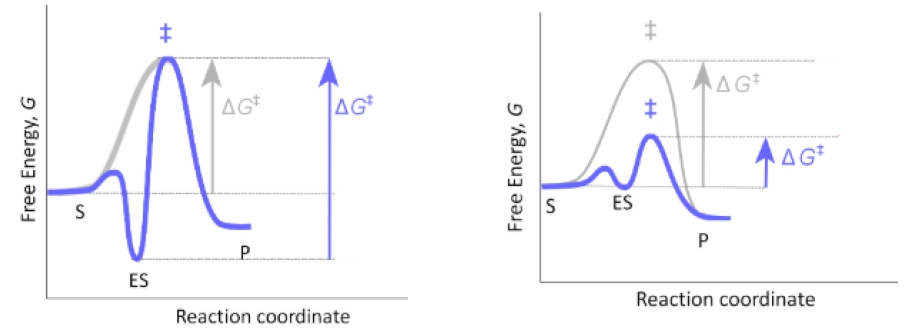
So in our last lesson video, we said that the lock and key model is a less likely model for enzyme substrate specificity. A more likely model for enzyme substrate specificity is the induced fit model, which is what we're going to talk about in this video. So with the induced fit model, unlike the lock and key model, the shape of the active site on the enzyme is not rigid. It's actually adjustable. Instead of being perfectly complementary to the shape of the substrate, the active site's shape is actually more complementary to the transition state than it is to the shape of the substrate. Ultimately, this allows the enzyme to prioritize stabilizing the transition state over stabilizing the enzyme-substrate complex, like what happens with the lock and key model.
As we'll see down below in our example, with the induced fit model, there are conformational changes that take place and are induced in both the enzyme's active site and the substrate. If we take a look at our example of the induced fit model down below, notice that we have the enzyme over here in purple and then we have our square-shaped substrate over here in orange. Notice that with the induced fit model, the active site shape is not perfectly complementary to the shape of the substrate, and instead, the active site shape is more complementary to the transition state. When the substrate forms a complex with the enzyme, notice that there are conformational changes that are induced in both the enzyme's active site and the substrate.
So notice that the square-shaped substrate has changed conformations to this rectangular-shaped substrate. Also, notice that the enzyme's active site is more opened up and has essentially changed conformations. Ultimately, what happens when the enzyme-substrate complex forms in the induced fit model is that the enzyme-substrate complex is not stabilized like it was with the lock and key model. Instead, ultimately, what this means is that the transition state stabilization is prioritized with the induced fit model, and that is ultimately what makes the induced fit model a more likely model for enzyme catalysis. This again has to do with the fact that the enzyme-substrate complex is not stabilized like it is with the lock and key model. And when the transition state is stabilized and prioritized, ultimately what happens is there's a decrease in the energy of activation. And that's exactly what we want enzymes to do, and that's why the induced fit model is more likely.
If we take a look down below at our energy—this dotted line here that represents our uncatalyzed reaction, whereas the red line represents the enzyme-catalyzed reaction. And notice that the arrow here, the black arrow, represents the energy of activation for the uncatalyzed reaction, which is pretty large. And notice that this time the red arrow is representing the catalyzed energy of activation and it's actually smaller than the uncatalyzed energy of activation, which means that the reaction is going to be sped up. So the reason that we're able to get a smaller energy of activation for the catalyzed reaction is that notice that the enzyme-substrate complex is not as stabilized as it was with the lock and key model, and that allows for us to just stabilize the transition state and keep the energy of activation for the catalyzed reaction relatively small, instead of staying the same or potentially increasing, like it did with the lock and key model.
It's important to note, moving forward in our course, that the induced fit model is a more likely model for enzyme catalysis. That concludes our lesson on the induced fit model, and I'll see you guys in our next video where we'll be able to get a little bit of practice.
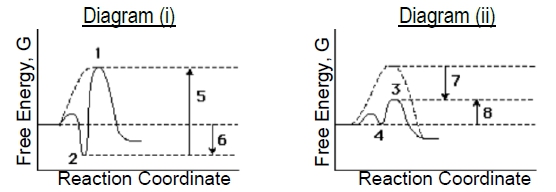
Compare the two enzyme-catalyzed reaction diagrams below (i & ii) to determine which of the following is true.
a) The ES-complex in diagram (i) is #2 and in diagram (ii) is #3..
b) Catalyzed EA in diagram (i) is arrow #5 and in diagram (ii) is arrow #7.
c) Binding energy in diagram (i) is arrow #5 and in diagram (ii) is arrow #7.
d) Diagram (i) describes a 'lock & key' model while (ii) describes more of an 'induced fit' model.
What is a potential disadvantage for an enzyme having too high of an affinity for its substrate?
Select the best option that fills in the blanks appropriately in the order of their appearance.
The left graph depicts an energy diagram for the '_____________' model of enzyme-substrate specificity, whereas the right diagram depicts an energy diagram for the modern '_____________' model. In the lock & key model, the enzyme binds tightly and precisely to the '_____________.' In the induced fit model, the enzyme binds weakly to the '_____________' and then changes conformation to bind tightly to the '_____________.'
Here’s what students ask on this topic:
What is the lock and key model in enzyme-substrate interaction?
The lock and key model describes enzyme-substrate interaction as a rigid fit, where the enzyme's active site is perfectly complementary to the substrate's shape. This model likens the enzyme to a lock and the substrate to a key, suggesting that only the correctly shaped substrate (key) can fit into the enzyme's active site (lock). This results in a stable enzyme-substrate complex. However, this model is less favored because it does not effectively lower the activation energy required for the reaction, which is a key function of enzymes.
 Created using AI
Created using AIWhy is the lock and key model considered less likely for enzyme catalysis?
The lock and key model is considered less likely for enzyme catalysis because it leads to an overly stable enzyme-substrate complex. This excessive stabilization does not lower the activation energy of the reaction, which is contrary to the primary role of enzymes. Enzymes are supposed to decrease the activation energy to speed up reactions. In the lock and key model, the activation energy remains the same or may even increase, making it an inefficient model for explaining enzyme activity.
 Created using AI
Created using AIWhat is the induced fit model in enzyme-substrate interaction?
The induced fit model describes enzyme-substrate interaction as a dynamic process where the enzyme's active site is flexible and adjusts its shape to fit the substrate. Unlike the lock and key model, the active site is more complementary to the transition state than to the substrate itself. This flexibility allows for conformational changes in both the enzyme and the substrate, leading to a less stable enzyme-substrate complex but a more stabilized transition state. This stabilization of the transition state effectively lowers the activation energy, making the induced fit model a more accurate representation of enzyme catalysis.
 Created using AI
Created using AIHow does the induced fit model lower the activation energy of a reaction?
The induced fit model lowers the activation energy of a reaction by prioritizing the stabilization of the transition state rather than the enzyme-substrate complex. When the substrate binds to the enzyme, both undergo conformational changes that make the active site more complementary to the transition state. This stabilization of the transition state reduces the energy barrier (activation energy) required for the reaction to proceed, thereby speeding up the reaction. This is in contrast to the lock and key model, where the enzyme-substrate complex is overly stabilized, failing to lower the activation energy effectively.
 Created using AI
Created using AIWhat are the main differences between the lock and key and induced fit models?
The main differences between the lock and key and induced fit models lie in the flexibility and stabilization of the enzyme-substrate complex. In the lock and key model, the enzyme's active site is rigid and perfectly complementary to the substrate, leading to a highly stable enzyme-substrate complex but failing to lower the activation energy. In contrast, the induced fit model features a flexible active site that adjusts to fit the substrate, prioritizing the stabilization of the transition state. This flexibility and transition state stabilization effectively lower the activation energy, making the induced fit model a more accurate representation of enzyme catalysis.
 Created using AI
Created using AI