In this video, we're going to calculate the isoelectric point of amino acids with ionizable R groups. It turns out that calculating the isoelectric point of amino acids with ionizable R groups is just a little bit more challenging than calculating the same for amino acids with non-ionizable R groups, and that's because amino acids with ionizable R groups have 3 pKa values instead of just 2 pKa values like those of amino acids with non-ionizable R groups. But the isoelectric point, or the pI, is still only an average of just 2 pKa values, and that's why it's a little bit more challenging because now we have to consider the following question: which pKa values do we need to average? So again, recall that amino acids with non-ionizable R groups only have 2 pKa values, so that makes it really easy to figure out which 2 pKa values we need to average to get the isoelectric point. But, with amino acids with ionizable R groups, they have 3 pKa values. So now we need to figure out which 2 of the 3 pKa values we need to average to get the isoelectric point. And we've got these 4 steps to help us figure out which pKa values to average.
And down below in our example, we have the same exact 4 steps just listed in a more visual way in a diagram or a flowchart. And so, when we go through each of the steps up above, if they don't quite click with you right away, hang on tight because once we get to our example and apply the steps in our example problem, the steps are going to make more sense to you. And as we get more and more practice utilizing the steps, the steps are going to make more and more sense.
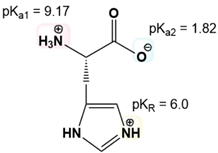
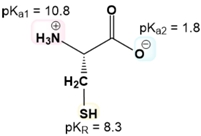
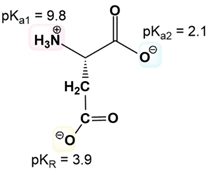
The first step is to just know the net charge of both the protonated and the deprotonated forms of ionizable R groups. The mnemonic is just "yucky crazy dragons eat knights riding horses." If you know this mnemonic, you know not only the 7 amino acids with ionizable R groups but also the nature of the ionizations in terms of having a positive or a negative charge when they are protonated or deprotonated.
Step number 2 is to order the 3 pKa values from smallest to largest and then to use the middle pKa as a guide in step number 3. This will make more sense once we get to our example down below.
The third step is to determine the net charge of the predominant amino acid at any pH that we want as long as that pH falls between adjacent pKa values.
The fourth and final step is to average, of course, only 2 pKa values, the middle pKa from step number 2, as well as another pKa that needs to be determined. This pKa will be the one that's closest to the pH that gives an overall neutral net charge on the predominant structure from step number 3.
So in our example, it's asking us to calculate the isoelectric point of tyrosine, which has one ionizable R group. Given that tyrosine falls into our mnemonic, the Y here stands for "yucky" and is a negative word, telling us that Tyrosine's R group has a negative charge when it's ionized. Step number 1 is to know how the amino acids' R groups ionize. Just by knowing this mnemonic and knowing that Tyrosine's R group has a negative charge when it's ionized, step number 1 is complete.
Now, we are given these 3 pKa values for Tyrosine's 3 ionizable groups. The alpha amino groups have a ballpark pKa range from about 9 to 10.5. By identifying the pKr, we know it refers to the R group's pKa, which leaves the other values for the amino and carboxyl groups. We then order these pKa values from smallest to largest: pKa 2 of 2.2 for the carboxyl group, 9.1 for the amino group, and 10.1 for the R group. Step number 2 is complete.
Using the middle pKa, we determine the net charge of the predominant structure by considering pH values between the pairs of pKa values. We identify the correct region giving a net charge of 0 by comparing the pH to each pKa. Through this process, we find that the correct region to average is the middle pKa 9.1 and the carboxyl group pKa of 2.2. Averaging these pKas gives 9.1 + 2.2 / 2 = 5.65, which is the isoelectric point for Tyrosine, corresponding with answer option "d."
We'll be able to get a lot more practice in our next couple of videos, calculating the isoelectric point of amino acids with ionizable R groups. I'll see you guys in those videos.