Redox Reactions definitions Flashcards
 Back
BackRedox Reactions definitions
1/13
Terms in this set (13)
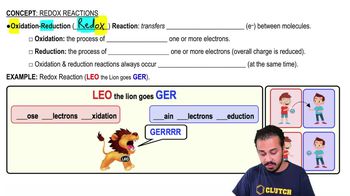
- Redox ReactionsInvolve the transfer of electrons between molecules, combining oxidation and reduction processes.
- OxidationThe process of losing one or more negatively charged electrons.
- ReductionThe process of gaining one or more negatively charged electrons, reducing overall charge.
- ElectronA negatively charged subatomic particle involved in redox reactions.
- NADHAn electron carrier that transports electrons and hydrogen ions, acting as a 'full taxicab'.
- FADH2An electron carrier that transports electrons and hydrogen ions, similar to NADH.
- NAD+The oxidized form of NADH, having fewer electrons and acting as an 'empty taxicab'.
- FADThe oxidized form of FADH2, having fewer electrons and acting as an 'empty taxicab'.
- Electron Transport ChainThe final stage of cellular respiration where electrons from NADH and FADH2 are transferred.
- Cellular RespirationA biological process involving redox reactions to produce energy, using electron carriers.
- Electron CarriersMolecules like NADH and FADH2 that transport electrons within cells.
- Hydrogen IonA positively charged ion that accompanies electrons in redox reactions.
- MnemonicA memory aid, such as 'Leo the lion goes ger', to remember redox concepts.