Properties of Water- Thermal definitions Flashcards
 Back
BackProperties of Water- Thermal definitions
1/15
Terms in this set (15)
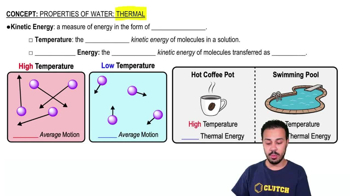
- Kinetic EnergyEnergy in the form of motion; substances in motion possess this type of energy.
- TemperatureAverage kinetic energy of molecules in a sample, indicating the average motion of the molecules.
- Thermal EnergyTotal kinetic energy of molecules transferred as heat, dependent on both temperature and volume.
- Specific HeatAmount of heat energy required to raise or lower the temperature of 1 gram of a substance by 1 degree Celsius.
- HomeostasisMaintenance of stable internal conditions in living organisms despite external environmental changes.
- Heat of VaporizationAmount of heat energy required to convert 1 gram of a liquid into its gaseous state.
- Hydrogen BondsStrong intermolecular forces between water molecules, contributing to water's high specific heat and heat of vaporization.
- EvaporationPhase transition from liquid to gas, requiring significant energy to break intermolecular bonds.
- Water VaporGaseous form of water, characterized by widely spaced molecules and absence of hydrogen bonds.
- Liquid WaterState of water where molecules are densely packed and hydrogen bonds are present.
- VolumeAmount of space occupied by a substance, influencing its thermal energy.
- Heat EnergyForm of energy transferred between substances due to temperature differences.
- MoleculesSmallest units of a chemical compound that can take part in a chemical reaction.
- Phase TransitionChange of a substance from one state of matter to another, such as from liquid to gas.
- Internal ConditionsStable conditions within an organism necessary for maintaining life processes.