Introduction to Water definitions Flashcards
 Back
BackIntroduction to Water definitions
1/10
Terms in this set (10)
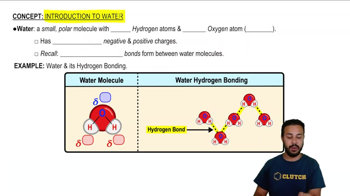
- Polar MoleculeA molecule with an uneven distribution of charges, resulting in partial positive and negative regions.
- Polar Covalent BondsA type of bond where electrons are shared unequally, leading to partial charges on atoms.
- Hydrogen BondsWeak attractions between the partial positive hydrogen of one molecule and the partial negative atom of another.
- CohesionThe attraction between molecules of the same substance, contributing to surface tension.
- AdhesionThe attraction between molecules of different substances, aiding in capillary action.
- Surface TensionThe elastic tendency of a fluid surface, allowing it to resist external force.
- Low Density in Solid FormCharacteristic of ice, where molecules are spaced further apart than in liquid water.
- High Specific HeatThe ability of water to absorb and retain heat, stabilizing temperatures.
- Heat of VaporizationThe energy required to convert water from liquid to gas, aiding in cooling.
- Universal SolventWater's ability to dissolve many substances due to its polarity, facilitating chemical reactions.