Introduction to Chemical Bonding definitions Flashcards
 Back
BackIntroduction to Chemical Bonding definitions
1/15
Terms in this set (15)
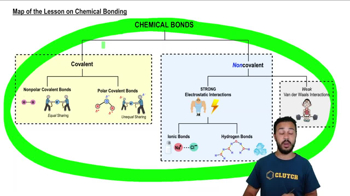
- Chemical BondsAttractive forces that hold atoms together to form molecules or compounds.
- MoleculesSubstances containing at least two chemically bound atoms, which can be of the same or different elements.
- CompoundsSpecific molecules composed of at least two different elements.
- Chemical FormulaNotation revealing the number and type of atoms in a molecule or compound.
- Intramolecular BondsInteractions between atoms within the same molecule.
- Intermolecular BondsInteractions between atoms of different molecules.
- Covalent BondsChemical bonds formed by the sharing of electron pairs between atoms.
- Nonpolar Covalent BondsCovalent bonds where electrons are shared equally between atoms.
- Polar Covalent BondsCovalent bonds where electrons are shared unequally, creating partial charges.
- Ionic BondsStrong electrostatic interactions between oppositely charged ions.
- Hydrogen BondsWeak bonds between a hydrogen atom and an electronegative atom.
- Van der Waals InteractionsWeak attractions between molecules due to temporary dipoles.
- Electrostatic InteractionsForces between charged particles, significant in ionic and hydrogen bonds.
- Oxygen GasA molecule consisting of two oxygen atoms chemically bound together.
- GlucoseA compound with the chemical formula C6H12O6, consisting of carbon, hydrogen, and oxygen.