26. Fluid and Electrolyte Balance, Acid Base Balance
Acid-Base Balance
26. Fluid and Electrolyte Balance, Acid Base Balance
Acid-Base Balance
Additional 12 creators.
Learn with other creators
Showing 15 of 15 videos
Practice this topic
- Multiple ChoiceNormal arterial blood pH is __________.1169views
- Multiple ChoiceThe most important buffer system in the intracellular fluid compartment (ICF) is the __________ buffer system.3259views
- Multiple ChoiceWhich statement about acids is true?1310views2rank
- Multiple ChoiceThe most important buffer system of extracellular fluid, such as plasma, is the __________ buffer system.2255views1rank
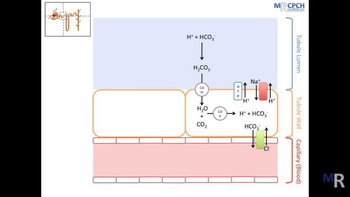
- Textbook QuestionAnswer questions 5 through 10 by choosing responses from the following: a. ammonium ions b. bicarbonate c. calcium d. chloride e. hydrogen ions f. magnesium g. phosphate h. potassium i. sodium j. water Two substances secreted into the proximal convoluted tubules in exchange for sodium ions.241views
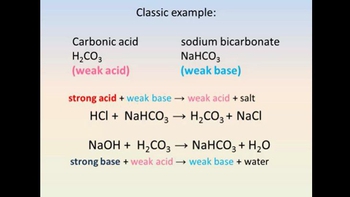
- Textbook QuestionMultiple Choice More than one choice may apply. In the carbonic acid–bicarbonate buffer system, strong acids are buffered by a. carbonic acid. b. water. c. bicarbonate ion. d. the salt of the strong acid.223views
- Textbook QuestionAnswer questions 5 through 10 by choosing responses from the following: a. ammonium ions b. bicarbonate c. calcium d. chloride e. hydrogen ions f. magnesium g. phosphate h. potassium i. sodium j. water Two ions produced during catabolism of glutamine.216views
- Textbook QuestionIn an individual with metabolic acidosis, a clue that the respiratory system is compensating is provided by a. high blood bicarbonate levels, b. low blood bicarbonate levels, c. rapid, deep breathing, d. slow, shallow breathing.322views