2. Cell Chemistry & Cell Components
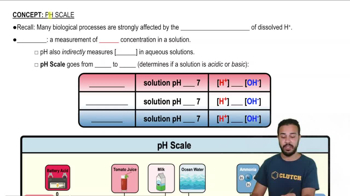
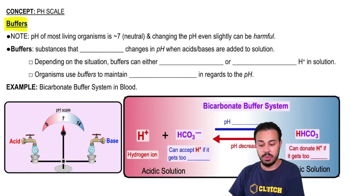
pH Scale
2. Cell Chemistry & Cell Components
pH Scale
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
In a neutral solution, the concentration of __________.
6660views50rank - Multiple Choice
A base _______:
4985views47rank - Multiple Choice
Which of the following statements about buffers is true?
5546views61rank - Multiple ChoiceA substance that minimizes changes in the concentration of H+ and OH– in a solution is a __________.2405views
- Textbook QuestionMeasurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?a. 4.0 Mb. 10−10Mc. 10−4Md. 104M2240views
- Textbook QuestionWhat is the hydroxide ion concentration of the lake described in question 3?a. 10−10Mb. 10−4Mc. 10−7Md. 10.0 M1785views
- Textbook QuestionA solution at pH 6 contains _________ H+ than the same amount of a solution at pH 8.a. 20 times moreb. 100 times morec. 2 times lessd. 100 times less1951views
- Textbook QuestionA can of cola consists mostly of sugar dissolved in water, with some carbon dioxide gas that makes it fizzy and makes the pH less than 7. In chemical terms, you could say that cola is an aqueous solution where water is the _________ , sugar is a _________ , and carbon dioxide makes the solution _________ .a. solvent . . . solute . . . basicb. solute . . . solvent . . . basicc. solvent . . . solute . . . acidicd. solute . . . solvent . . . acidic2088views