Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity of Water
Water is a polar molecule, meaning it has a partial positive charge on one side and a partial negative charge on the other. This polarity allows water to form hydrogen bonds, which are crucial for many of its unique properties, such as high surface tension and solvent capabilities. Understanding polarity is essential for grasping how water interacts with other substances.
Recommended video:
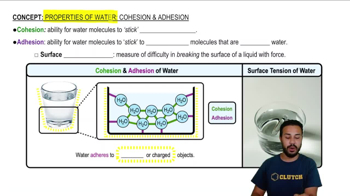
Cohesion and Adhesion
Cohesion refers to the attraction between water molecules, while adhesion is the attraction between water molecules and other substances. These properties are vital for processes like capillary action, which allows water to move through plants and soil. Recognizing these concepts helps explain water's role in biological systems and ecosystems.
Recommended video:
Properties of Water- Cohesion and Adhesion
Specific Heat Capacity
Water has a high specific heat capacity, meaning it can absorb a lot of heat without a significant change in temperature. This property is important for regulating climate and maintaining stable temperatures in organisms and environments. Understanding specific heat capacity is crucial for appreciating water's role in supporting life and influencing weather patterns.
Recommended video:
Water’s High Specific Heat
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution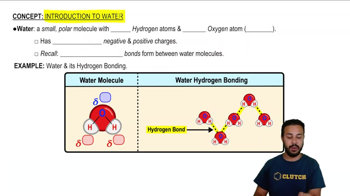


 5:19m
5:19m